Nomograms based on HPV load for predicting survival in cervical squamous cell carcinoma: An observational study with a longterm follow-up
Jing Zuo, Ying Huang, Jusheng An, Xi Yang, Ning Li, Manni Huang, Lingying Wu
1Department of Gynecologic Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China;
2Women’s Health Integrated Research Center, the Henry Jackson Foundation, Annandale, VA 22003, USA
Correspondence to: Prof. Lingying Wu, MD. Department of Gynecologic Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China. Email:wulingying@csco.org.cn.
Abstract Objective: To investigate the prognostic value of pretreatment human papillomavirus (HPV) viral load for cervical cancer, and to develop nomograms based on HPV load and other clinicopathological factors for long-term survival.Methods: We conducted a prospective study on cervical squamous cell carcinoma (SCC) patients diagnosed between January 2003 and December 2008. Cervical samples were tested for HPV viral load by the Hybrid Capture II (HCII) assay before treatment and 6 months after treatment. Clinical characteristics and follow-up information were also collected. A multivariable Cox proportional hazards model was used to adjust covariates in both the radical hysterectomy (RH) treatment group and concurrent chemoradiotherapy (CCRT) treatment group to identify relevant covariates, and then nomograms were constructed and used for internal validation.Results: A total of 520 SCC patients enrolled in this study with a median follow-up of 127 months, 360 patients received RH, whereas 160 patients received CCRT. The median HPV viral load in RH and CCRT groups was 356.10 and 294.29, respectively. Tumor size was positively correlated with high pretreatment HPV load in both groups. In CCRT group, the advanced International Federation of Gynecology and Obstetrics (FIGO) stage and enlarged retroperitoneal lymph node status determined by computed tomography (LNSCT) were correlated with low HPV load group. Initial HPV viral load, FIGO stage and lymph node metastasis were prognostic factors for RH group, whereas HPV viral load, squamous cell carcinoma antigen (SCC-Ag) level and LNSCT were identified as prognostic factors for CCRT group. Nomograms incorporating these predictors for 10-year progression-free survival (PFS) were constructed [concordance index (C-index): 0.756, 0.749].Conclusions: A low pretreatment HPV viral load is an independent prognostic factor for poor prognosis of cervical SCC and is related to other clinicopathological factors. The survival nomogram based on HPV viral load could predict the long-term prognosis.
Keywords: Cervical squamous cell carcinoma; human papillomavirus; viral load; survival nomogram
Introduction
Cervical cancer is the third most common cause of cancerrelated mortality among females in developing countries,which caused 144,400 deaths in Asia in 2012 (1). In 2014,the estimated number of new cases and deaths from cervical cancer in China were 102,000 and 31,000 respectively (2).Persistent cervical high-risk human papillomavirus (HRHPV) infection is an etiological factor for the development of cervical cancer and its precursors (3). Two widely used efficient measurements to detect the presence of HPV DNA in the cervix are the Hybrid Capture II (HCII) assay and the polymerase chain reaction (PCR). However, the former that detects semiquantitative viral load of accumulation of one or more of the 13 types of HR-HPV is more widely used in developing countries for its reliability.After nearly two decades of continuous research, the role of the high-risk HPV viral load in the progression of cervical lesions has been clearly established, and it has been accepted that the viral load rises when disease severity increases in cervical lesions; viral load has shown an increasing tendency with the progression from normal to invasive squamous cell carcinoma (SCC) (4,5).Furthermore, a high viral load might predict the persistence or recurrence of cervical intraepithelial neoplasia (6).
Although several studies have demonstrated a relationship between the HPV viral load and the outcome of cervical cancer patients who underwent concurrent chemoradiotherapy (CCRT) or radical hysterectomy (RH)(7,8), the clinical value of viral load for predicting outcome in cervical cancer remains controversial (9) because of the variation in sampling techniques and the different methods used to calculate viral load. We conducted a prospective observational study with a long-term follow-up to evaluate the prognostic significance of HPV viral load in cervical SCC, further to develop a proper applicable prognostic nomogram based on HPV load for the estimation of outcomes and to validate its predictive capacity in the independent cohort.
Materials and methods
Case selection
Patients with histologically confirmed cervical SCC who was staged as International Federation of Gynecology and Obstetrics (FIGO) stage IB1-IVA undergoing primary treatment in the Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) from January 2003 to December 2008 were eligible for the study. The eligibility criteria included the following: 25-75 years old; World Health Organization (WHO) performance status of two or less;adequate bone marrow reserve; and adequate renal, hepatic and cardiac function. The exclusion criteria included mixed histological cervical cancer or patients with prior cancer treatment. The tumor stage was determined by a pelvic examination by two gynecological oncologists according to FIGO stage 1994 edition. The status of the retroperitoneal lymph nodes and major organs was evaluated by computed tomography (CT) to exclude distant metastases. The pretreatment evaluation also included squamous cell carcinoma antigen (SCC-Ag) and HPV HCII testing. The study was approved by the Ethics Board of the Cancer Hospital, CAMS. Informed consent was obtained from all patients.
Treatment and follow-up
Patients with stage IB1-IIA disease were treated with RH and retroperitoneal lymphadenectomy. Patients with risk factors, such as lymph node metastasis, parametrial invasion, positive surgical margins, vaginal involvement,deep stromal invasion (>2/3) and large tumor size (>4 cm)were referred for postoperative adjuvant treatment with whole pelvic external beam radiotherapy (EBRT; total dose, 45-50 Gy) or CCRT with 4-6 weeks of cisplatin (40 mg/m2) administered weekly by intravenous injection.
Patients with stage IIB-IVA were treated with CCRT consisting of whole pelvic EBRT and high-dose-rate brachytherapy (HDR-BRT). A total EBRT dose of 45-50 Gy was delivered 5 d per week. The volume of EBRT covered the region of gross disease, the parametrial and uterosacral ligaments, and sufficient vaginal margin of at least 3 cm from the region of gross disease as well as all pelvic nodal volumes at risk. For patients with pelvic lymph node involvement, the common iliac lymph nodes were covered. In patients with common iliac and/or para-aortic nodal involvement, extended-field pelvic and para-aortic radiotherapy was delivered. A boost irradiation dose of 10-15 Gy was delivered to enlarged nodes. All patients received 4-6 weeks of cisplatin (40 mg/m2) administered weekly by intravenous injection, which was utilized as the concomitant chemotherapy regime during EBRT. HDRBRT was performed once a week with a daily dose of 7 Gy at point A following 30-40 Gy delivered by EBRT. All patients received a total cumulative dose to point A of at least 85 Gy (equivalent dose in 2 Gy/f, EQD2).
After the primary treatment, all patients were followed up at every 3 months during the first and second years and every 6 months in the third to fifth years. After 5 years, the patients were reviewed once per year. Follow-up period up to June 2018. Disease status was assessed by history taking,physical examination, vaginal cytology and appropriate laboratory and imaging examination [CT/magnetic resonance imaging (MRI)]. Suspected recurrent disease was confirmed with a biopsy whenever possible.
Hybrid capture II and SCC-Ag assay
Specimens were collected within 2 weeks before the initiation of treatment and 6 months after the completion of treatment. The cytology sample was taken from the cervix or vaginal cuff with a Cytobrush (Digene Cervical Sampler; Digene Corp., Gaithersburg, MD, USA). The presence of HPV DNA in the mucosal exfoliated cells was determined by the HCII assay (Digene Cervical Sampler;Digene Corp.). The DNA specimens were denatured and hybridized with a cocktail of full-length RNA probes directed against a panel of 13 high-risk HPVs (HPV 16, 18,31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). Compared with a standard containing 1.0 pg/mL HPV-16 DNA(about 5,000 viral copies), the positive cutoff is equal to the mean of three positive control values. Specimens with relative light unit/cutoff (RLU/CO) ratios ≥1.0 were regarded as positive; otherwise regarded as negative. All HCII results were divided into two categories: viral load not more than the median value was assessed as a low viral load; and viral load greater than the median value was assessed as a high viral load.
Blood samples were collected preoperatively from all participants. The SCC-Ag levels were performed using the following analyzers in serum sample. Samples from January 2002 to January 2007 were tested using the IMx immunoassay automation system for ion capture immunoassay (ICIA), while samples from February 2007 till June 2018 using the Architect i2000SR Chemical luminescence immunity analyzer (CLIA) (Abbott Laboratories, Abbott Park, IL, USA). The cutoff value was 1.5 ng/mL for both two methods. The SCC-Ag greater than 1.5 ng/mL was assessed as abnormal high level.
Statistical analysis
The endpoints of the present study were overall survival(OS) and progression-free survival (PFS). PFS was defined as the time from study entry to the date of physical,radiographic or histological evidence of disease recurrence,death by any cause, or the most recent follow-up,whichever came first. OS was defined as the time from study entry to the date of death by any cause or the most recent follow-up, whichever came first. Since the treatment of recurrence cervical cancers has evolved during the past 16 years, which would influence the survival, we chose 10-year PFS to establish nomograms.
The data were analyzed with SAS software (Version 9.4;SAS Institute Inc., Cary, USA) and the Hmisc, rms package in R software (Version 3.0.2; R Foundation for Statistical Computing, Vienna, Austria). All P values reported are two-sided, and P<0.05 was considered statistically significant. The HPV viral loads were categorized into two groups (low vs. high) according to the median load. The distributional differences between the HPV viral groups were assessed using Pearson’s χ2test or Fisher’s exact test.The Kaplan-Meier method and the log-rank test were used to compare the PFS and OS between subgroups. The PFS and OS curves were generated using the Kaplan-Meier method. SCC-Ag level was modeled as continuous variables, and other variables were modeled as categorical variables. The hazard ratios (HRs) and 95% confidence intervals (95% CIs) for these variables were calculated with a Cox proportional hazards model. A multivariate Cox regression model was used to develop a prediction model for the 10-year PFS. The nomogram for both the RH group and the CCRT group was constructed based on the Cox model parameter; we identified clinical features that have been demonstrated to be associated with survival, and incorporated these as prognostic features. Nomogram construction and validation were performed with Iasonos’guide (10), with a concordance index (C-index) being estimated by analyzing the area under the curve of the receiver operating characteristic curve. Next, a calibration plot was constructed to determine whether the observed probabilities for survival were in concordance with predicted probabilities for survival. Bootstrap resampling(1,000 resamples) was used for this plot.
Results
Patients’ characteristics
During the study period, 556 patients who met the inclusion criteria were identified. Twenty-seven patients were lost to follow-up, and nine patients had non-cervical cancer-related deaths during the follow-up period, which left 520 patients eligible for evaluation, with the loss of follow-up rate 4.93% (95% CI, 0.031-0.068). The enrolled patients accounted for 27.7% (520/1,880) of all patients receiving initial treatment during the same period at CAMS. Supplementary Table S1 shows that the enrolled patients well represent the clinicopathologic features of the initial treated patients. In the current study, the median age of the 520 patients was 43 (range, 23-74) years. A total of 360 patients received RH (+/- adjuvant therapy),accounting for 42.6% of all RH patients, while 160 patients received CCRT (+/- adjuvant chemotherapy), accounting for 15.5% of all CCRT patients. The median follow-up for the enrolled patients was 127 (range, 11-180) months. The major clinicopathological characteristics of RH and CCRT groups are summarized in Table 1.
HPV viral load and its relationship with clinicopathological factors
In the pretreatment test, 358 (99.44%) patients were HPV positive in RH group. The median HPV viral load was 356.10 (range, 0.89-3,215.23). In the CCRT group, 159 patients (99.38%) were HPV positive, and the median HPV viral load was 294.29 (range, 0.36-2,938.23). The negative results were grouped into the low HPV viral load group. In the 6-month posttreatment test, 150 of the 360 patients were still HPV positive in the RH group, with the median HPV load of 0.85, while 74 of the 160 patients were still HPV positive in CCRT group, with the median HPV load of 0.96.
The initial HPV viral load showed a correlation with several clinicopathologic parameters. Tumor size was positively correlated with high pretreatment HPV load.The diameter of tumor larger than 2 cm was associated with high HPV load in the RH group (P=0.002), whereas in the CCRT group, the tumor diameter larger than 4 cm was associated with high HPV load (P=0.024). In the advanced patients (CCRT group), as FIGO stage increased,the number of patients in the low HPV load group was also significantly increased as compared to that in high HPV load group (P=0.020). Furthermore, the enlarged retroperitoneal lymph node status determined by computed tomography (LNSCT) was correlated with low viral load(P=0.030) (Table 2). However, neither FIGO stage nor lymph node status in the RH group was associated with viral load, nor were SCC-Ag and histologic grade in both groups.
Treatment failure pattern
In total, 86 patients had disease progression during followup. There were 41 (11.4%) cases of recurrence in the RH group, including 11 distant metastases, 24 regionalrecurrences and 6 local recurrences. The CCRT group had 45 (28.1%) cases of recurrence, including 22 distant metastases (4 patients experienced both local and distant recurrence), 15 regional recurrences and 8 local recurrences.

Table 1 Clinicopathological characteristics of RH and CCRT groups
HPV viral load and PFS/OS
The OS rate of RH and CCRT groups in follow-up period was 90.0% and 71.7%, respectively. According to the univariate analysis, low initial HPV viral load, SCC-Ag,FIGO stage, deep cervical stromal invasion, lymph node metastasis, tumor size and postoperative adjuvant treatment were associated with poor PFS in the RH group (Table 3).In the CCRT group, low viral load and other factors including initial high SCC-Ag level, LNSCT were associated with poor PFS (Table 4).
The multivariate Cox analysis identified that HPV viral load, FIGO stage and lymph node metastasis were significantly correlated with PFS and OS in the RH group(Table 3), whereas in the CCRT group, HPV viral load,and LNSCT were found to be independent prognostic factors for PFS and OS, but pretreatment SCC-Ag was only an independent prognostic factor of PFS not for OS(Table 4). The Kaplan-Meier survival plots showed significant differences in PFS and OS when stratified by median pretreatment HPV viral load (Figure 1).
The independent prognostic factors based on the multivariate Cox analysis were used to create 10-year PFS nomograms for both RH and CCRT groups. Thenomogram for RH patients is shown in Figure 2A, and the nomogram for CCRT patients is shown in Figure 2B. The predictive accuracy for 10-year PFS as measured by the Cindex was 0.756 for RH group and 0.749 for CCRT group by the internal validation. The calibration plot for the probability of 10-year PFS in RH group and CCRT group is shown in Figure 3. There was a good correlation between the actual observed outcome and the prediction by nomograms. The sum of each variable value was plotted on the total point axis, and the estimated median 10-year PFS rates were obtained by drawing a vertical line from the plotted total point axis straight down to the outcome axis.
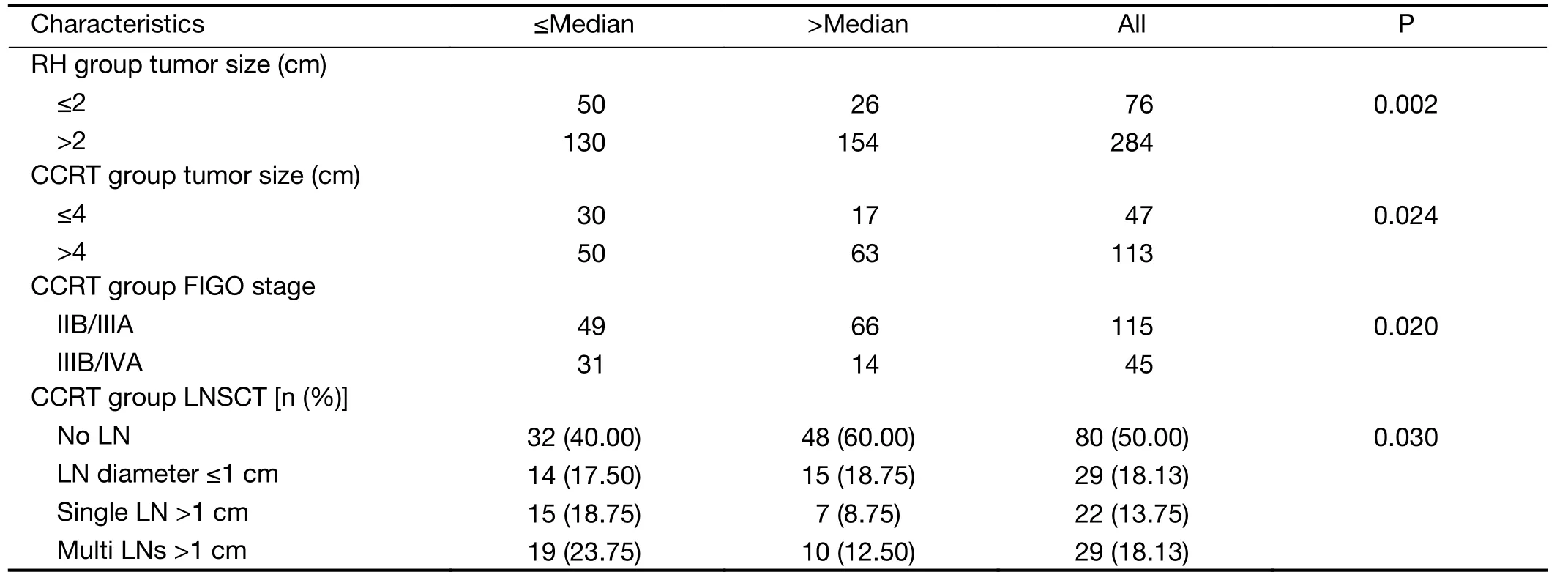
Table 2 Association between HPV load and clinicopathological characteristics
Discussion
In the current study, we illustrated that a low pretreatment HPV viral load was significantly independently related to unfavorable PFS and OS by two independent cohorts,which well represented the features of the patients in the period. Furthermore, we successfully incorporated the recognized risk factors to construct two novel nomogram models to predict long-term survival.
Our findings of predictive value of HPV viral load have been consistent with previous studies. Deng and Song respectively elaborated that the low viral load was associated with poor disease-free survival (DFS) in RH patients and local recurrence-free survival in CCRT patients, while Deng explained why the low HPV viral load was correlated with poor DFS in RH patients. Applying the
HCII and PCR genotyping simultaneously, Kim not only identified that the low HPV viral load was correlated with poor DFS in CCRT patients, but also found that initial HPV viral load was strongly associated with HPV genotype(7,8,11). We expanded those previous works and concurrently elaborated that low HPV viral load was also correlated with worse survival in patients receiving two mainstay treatments in different stages of disease. In addition, with the advantage of longer follow-up period,our study indicated that the viral load was correlated with not only PFS, but also poor OS.
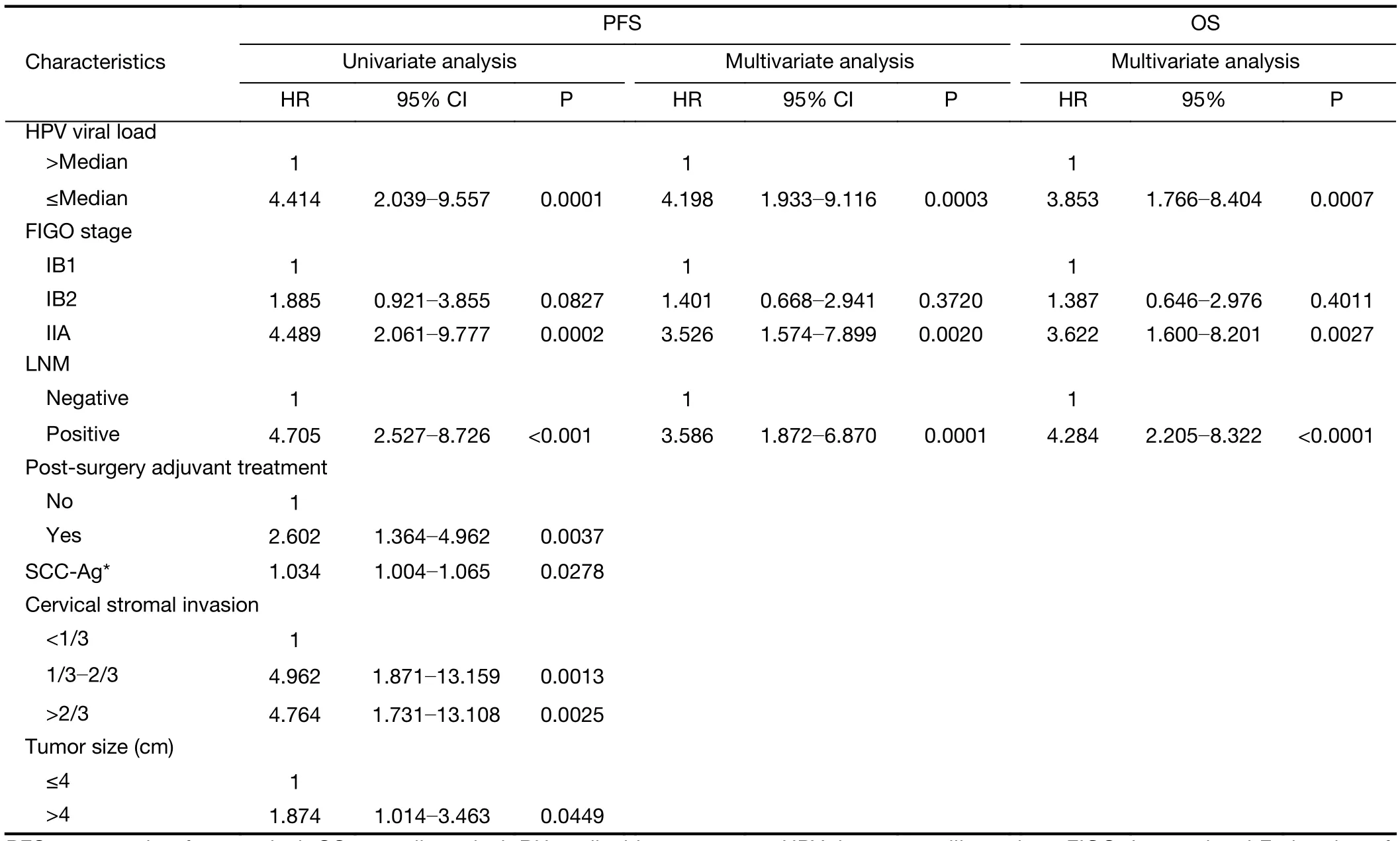
Table 3 Univariate and multivariate analyses of PFS and multivariate analyses of OS in RH group
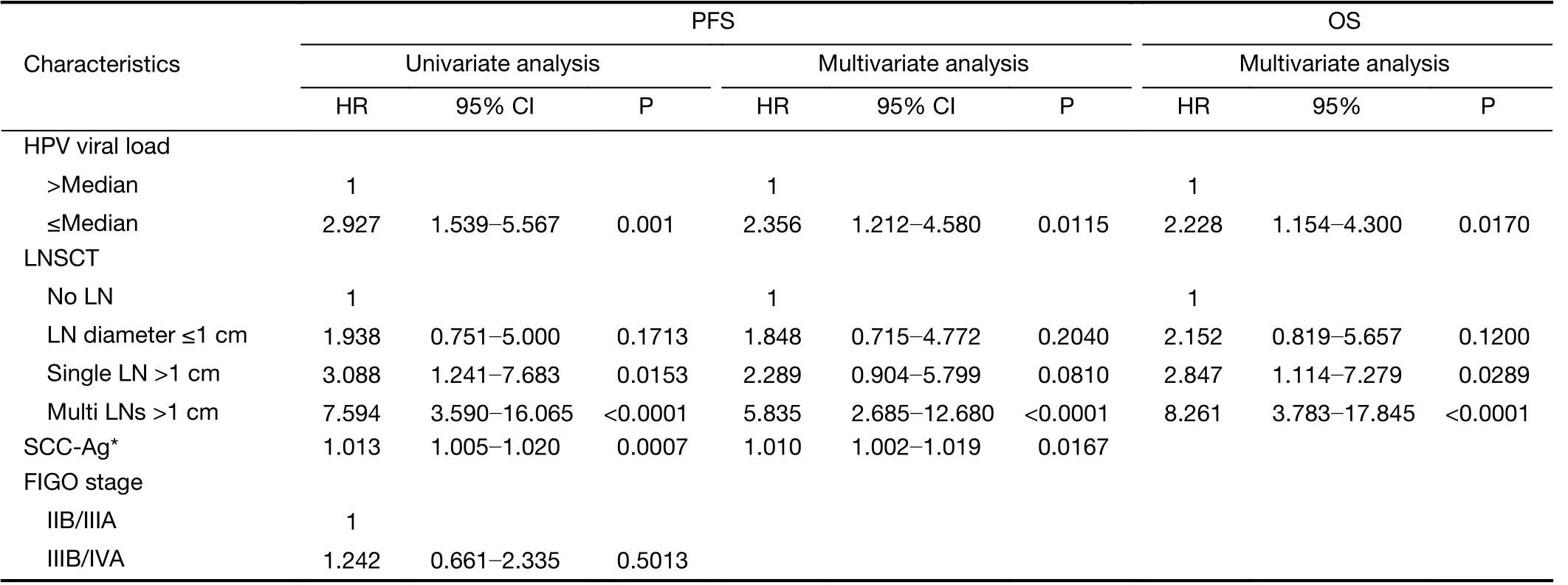
Table 4 Univariate and multivariate analyses of PFS and multivariate analyses of OS in CCRT group
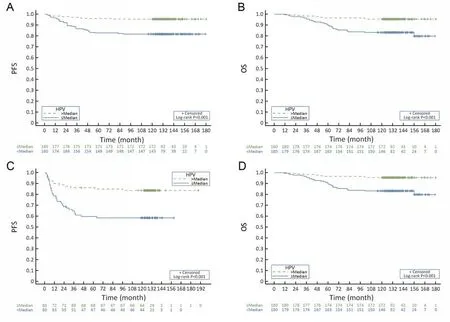
Figure 1 Kaplan-Meier survival plots in progression-free survival (PFS) and overall survival (OS) stratified by median pretreatment human papillomavirus (HPV) viral load. (A) PFS by HPV viral load in the radical hysterectomy (RH) group (P<0.001); (B) OS by HPV viral load in the RH group (P<0.001); (C) PFS by HPV viral load in the concurrent chemoradiotherapy (CCRT) group (P<0.001); (D) OS by HPV viral load in the CCRT group (P<0.001).
As we know, the integration of the viral genome represents a critical event in cervical carcinogenesis. The integration of the E6, E7 and long control region HPV genes into the host genome and the disruption of the open reading frame of the E1 or E2 gene result in the overexpression of E6 and E7 and concurrently cause a reduction in the viral load. The integration further interferes with the function of the tumor suppressor p53 and pRb, and therefore, eventually leads to proliferation and malignant transformation of infected epithelial cells(12). The aforesaid etiology provides a theoretical basis for our results, Other relevant studies also indirectly confirmed the prognostic significance of low load through HPV typing and integration status (4,13,14).
To our knowledge, this prospective observational study led to the first nomogram constructed based on HR-HPV viral load, and we enrolled the largest sample size of consecutive patients with the longest follow-up period to verify the prognostic value of HPV load.

Figure 2 Nomograms for predicting 5-year and 10-year progression-free survival (PFS) in radical hysterectomy (RH) group (A) and concurrent chemoradiotherapy (CCRT) group (B). The nomogram was constructed by incorporating three predictors. Find patient’s human papillomavirus (HPV) viral load on HPV viral load axis, then draw straight line upward to points axis to determine how many points for HPV viral load. Repeat the process for each axis, each time drawing straight line upward toward points axis. Sum points received for each predictor, and find sum on total points axis. Draw straight line down to survival-probability axis to calculate the 5-year and 10-year PFS probability. FIGO, International Federation of Gynecology and Obstetrics; SCC-Ag, squamous cell carcinoma antigen; CT, computed tomography; LN, lymph node.

Figure 3 Calibration curves for nomogram 10-year progression-free survival (PFS) predictions in radical hysterectomy (RH) group (A) and concurrent chemoradiotherapy (CCRT) group (B). Dashed line represents the ideal nomogram; solid line represents the performance of current nomogram. Solid dots indicate nomogram-predicted probabilities, vertical bars indicate 95% confidence intervals (95% CIs), and crosses indicate bias-corrected estimates.
The FIGO staging is the present standard for risk assessment and reflects the strongest prognostic parameter in patients with cervical cancer (15). However, stage alone is not sufficient to predict the survival. Other risk factors for recurrence have been identified: for operable earlystage patients, the high-risk factors are lymph node metastasis, positive resection margin and parametrial involvement (16), whereas for the advanced patients received CCRT, the age, performance status, para-aortic and pelvic lymph node status and tumor size were independent prognostic factors (17). Multiple nomogram models for the prediction of recurrence, distant metastasis and survival in RH or CCRT patients have been developed in recent years (18-24). Our nomogram introduced the HPV load as the new parameter. Despite the cutoff value(the median viral load in the cohort) is subject to variation among many external factors, our cutoff value is similar to those in previous studies, which greatly increase the feasibility. Furthermore, it’s worth noting that some common risk factors were excluded in the nomograms,which emphasized the impact of HPV viral load. For instance, few patients had positive vaginal margins (12/360,3.3%), while no patients with parametrial involvement were found in RH group. Thus, margin status and parametrial involvement that showed little prognostic significance in Cox analysis were not included in the nomograms. Moreover, in CCRT group, as the majority of patients (69.3%) had IIB stage disease, it was not a surprise that the FIGO stage was not an independent prognostic factor in CCRT group, which was also excluded from nomograms.
In addition to low HPV viral load, we recognized that initial high SCC-Ag level was the independent prognostic factor of PFS only in CCRT group, which was attributed to 71.9% (115/160) of patients with initial abnormal SCCAg and supported by two systematic-analyses (25,26).
The nomograms built on our cohort were internalvalidated for 5-year and 10-year PFS. The predictive accuracy measured by the C-index was as high as 0.756 for RH group and 0.749 for CCRT group. Moreover, our nomograms discriminated for treatment outcomes had excellent calibration ability, and therefore, it is applicable to estimate a more individualized prognosis based on individual risk factors for patients who met the eligibility criteria of this study. In particular, cervical cancer patients who are lacking other risk factors would benefit greatly from our nomograms that predict the possibility of longterm survival through HPV load.
Although we found positive relationship between low HPV viral load and poor prognosis in both RH and CCRT group, and have constructed the long-term PFS nomograms, there are limitations in our study. First, due to the semiquantitative nature of HCII, median HPV viral load is selected as the cutoff. Its predictive efficacy has been questioned. In the future, the relationship between subtype viral load and prognosis still needs to be verified by quantitative reverse transcription-polymerase chain reaction (RT-PCR). Second, we have not further validated the model with our own data, and thus external validation should be conducted with multicenter data.
Conclusions
A low HPV viral load is associated with other prognostic factors in both RH group and CCRT group. The multivariable analysis identified that a low pretreatment HPV viral load was an independent prognostic factor for worse prognosis in cervical cancer patients who underwent two mainstay treatments. We established nomograms for predicting prognosis based on the association of viral load with other factors.
Acknowledgements
This study was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2016-I2M-1-001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2019年2期
Chinese Journal of Cancer Research2019年2期
- Chinese Journal of Cancer Research的其它文章
- Alisol B 23-acetate-induced HepG2 hepatoma cell death through mTOR signaling-initiated G1 cell cycle arrest and apoptosis: A quantitative proteomic study
- Sequential therapy according to distinct disease progression patterns in advanced ALK-positive non-small-cell lung cancer after crizotinib treatment
- Efficacy of endoscopic treatment on patients with severe dysplasia/carcinoma in situ of esophageal squamous cell carcinoma: A prospective cohort study
- Histogram analysis of apparent diffusion coefficient predicts response to radiofrequency ablation in hepatocellular carcinoma
- Weekly albumin-bound paclitaxel/cisplatin versus gemcitabine/cisplatin as first-line therapy for patients with advanced non-small-cell lung cancer: A phase II open-label clinical study
- Synthesis and evaluation of 64Cu-radiolabeled NOTA-cetuximab(64Cu-NOTA-C225) for immuno-PET imaging of EGFR expression
