Retrospective evaluation of FOLFlRl3 alone or in combination with bevacizumab or aflibercept in metastatic colorectal cancer
Madeline Devaux, Laura Gerard, Corentin Richard, Leila Bengrine-Lefevre, Julie Vincent, Antonin Schmitt,Fran?ois Ghiringhelli
Abstract
Key words: Colorectal cancer; Chemotherapy; Irinotecan; Second-line; Aflibercept
INTRODUCTION
Metastatic colorectal cancer (mCRC) is a common disease in western countries[1]. In the absence of resection of all metastatic and primary tumours, the treatment of mCRC remains palliative. The standard of care involves chemotherapeutic protocols that include fluoropyrimidine in combination with oxaliplatin or irinotecan. Anti EGFR mAb and antiangiogenic drugs such as bevacizumab and aflibercept can be used in combination with chemotherapy to improve response rate, progression free survival and overall survival (OS)[2]. Recently, regorafenib and TAS-102 were developed as new therapeutic options upon failure of classical chemotherapeutic regimens[3,4]. In most mCRC patients, doublet chemotherapy using fluoropyrimidinebased chemotherapy with either irinotecan (FOLFIRI) or oxaliplatin (FOLFOX) in combination with anti EGFR or an antiangiogenic agent is considered the standard first-line of treatment. Second-line drug selection mainly depends on the regimen used in the first-line chemotherapy. Chemotherapy is frequently used in combination with antiangiogenic agents (bevacizumab, aflibercept, ramucirumab) or anti-EGFR agents when theRASmutation is present[5-10].
Previous reports proposed that the standard FOLFIRI regimen could be optimized by splitting the dose of irinotecan into two days. Half of the total dose is administered on day 1 prior to 5-FU dosing and the other half of the dose is administered on day 3 after 5-FU dosing. This protocol was named FOLFIRI3 regimen[11]. Despite their similarities, the FOLFIRI3 regimen benefits from an increased response rate compared to the classical FOLFIRI regimen[11,12]. A previous report suggests that FOLFIRI3 plus bevacizumab could also be used to improve the response rate and overcome resistance to previous treatment with FOLFIRI[13].
Recently, aflibercept was approved as a second-line chemotherapy in combination with FOLFIRI for mCRC patients whose cancer progressed after oxaliplatin based chemotherapy. This treatment is a new second-line chemotherapeutical option in addition to the previously established[8], but association with of aflibercept with FOLFIRI3 was not reported and compared with FOLFIRI3 or FOLFIRI3 plus bevacizumab.
In this retrospective study, we report a large cohort of patients treated with the FOLFIRI3 regimen and compare the safety and efficacy of FOLFIRI3 alone and in combination with bevacizumab or aflibercept.
MATERIALS AND METHODS
Study design
This study was a retrospective, monocentric study performed at Centre Georges Fran?ois Leclerc, Dijon France.
Participant
Study includes all consecutive patients treated with the FOLFIRI3 regimen for mCRC in our centre. From January, 2008 to December, 2017, patients were identified through the chemotherapy prescription computer software programme used at the cancer centre (CHIMIO?, Computer Engineering). The database was declared to the National French Commission for bioinformatics data and patient liberty (CNIL). The study was performed in agreement with French regulations with approval from the local institutional review boards. A general informed consent was signed by all cancer patients at the time of their first hospitalization in the cancer centre, enabling patient clinical and biological data analysis in this cohort study. Demographics, cancer history, toxicity according to the Common Toxicity Criteria [Common Toxicity Criteria (CTC) v2.0 (http://cancer.gov/)], and treatment outcomes, as well as pathological, clinical, biological, and radiological data [tumour response according to the Response Evaluation Criteria in Solid Tumours (RECIST) v1.1 criteria], were retrospectively collected from medical records. To be evaluable, all patients must have received at least four cycles of chemotherapy. Patients were classified as follows:complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). For statistical analysis, the best tumour response was selected. Patients with either CR, PR, or SD were classified as responders and patients with PD as nonresponders.
Settings
Patients were treated with bevacizumab at a dose of 5 mg/kg on day 1 every two weeks. The FOLFIRI3 regimen was given every 14 d as follows: on day 1, irinotecan 100 mg/m2 as a 1-h infusion, running concurrently with leucovorin 200 mg/m2as a 2-h infusion via a Y-connector, followed by 5-FU 2000 mg/m2as a 46-h infusion using an electric pump. On day 3, irinotecan 100 mg/m2as a 1-h infusion was repeated, at the end of the 5-FU infusion. Bevacizumab was given as a 30 min infusion every 2 wk at 5mg/kg. Aflibercept was given as a 1-h infusion every 2 wk at 4 mg/kg.
Statistical analysis
All patients were followed until death, loss to follow-up, or termination of the study(or whichever occurred first). The objective response rate (ORR) was defined as the proportion of patients having either a CR or PR according to RECIST version 1.1. The disease control rate (DCR) was defined as the percentage of patients who achieved CR, PR or SD. Progression-free survival (PFS) was defined as the time between the treatment start date and the date of disease progression or death from any cause.Patients who were alive without PD at the time of the final analysis were censored. OS was defined as the time between the date and the date of patient death from any cause or to the last date the patient was known to be alive. Patients still alive at the time of the analysis were excluded. Disease characteristics were examined using theχ2test or Fisher’s exact test for qualitative variables and the Kruskal-Wallis rank sum test for continuous variables, as appropriate. Univariate and multivariate survival analyses were performed using the Cox regression model. Survival probabilities were estimated using the Kaplan-Meier method. OS and PFS medians were calculated with the reverse Kaplan-Meier method and survival curves were compared using the logrank test. Patients were categorized into one of two cohorts according to their irinotecan status (irinotecan-na?ve and those who were previously treated with irinotecan). Patients were also categorized into one of three cohorts according to the treatment regimen they received: FOLFIRI3 alone, FOLFIRI3 plus bevacizumab and FOLFIRI3 plus aflibercept. Data analysis was performed using the statistical software R (http://www.R-project.org/) and representations were made with Prism 7(GraphPad, San Diego, CA, United States). All tests were two-sided, andP-values <0.05 were considered statistically significant.
Availability of data and materials
The clinical datasets collected and/or analyzed during the current study are available from the corresponding author on reasonable request.
RESULTS
Patient characteristics
Between January 2008 and December 2017, a total of 153 patients received at least one injection of the FOLFIRI3 regimen at the Department of Medical Oncology, Georges-Francois Leclerc Cancer Centre, Dijon, France. Eighteen received the FOLFIRI3 regimen, 99 received bevacizumab plus the FOLFIRI3 regimen and 36 received aflibercept plus FOLFIRI3. The main clinical characteristics of patients included in this retrospective study are shown in Table 1. The study included 84 males and 69 females and median age was 64 years (range 33-86). The performance status of this population was good with only 14% of patients having an ECOG performance status of 2. Only 29% of the patients had a right-side tumour. RAS and/or BRAF mutations were observed in 53% of the assessable samples. All patients had previously received at least one line of systemic chemotherapy. Only 28 (18%) of the patients were irinotecan-na?ve. 28% of the patients previously received bevacizumab, and 30% were previously treated with an EGFR therapy. Patients receiving either FOLFIRI3,bevacizumab plus FOLFIRI3 or aflibercept plus FOLFIRI3 did not differ in their clinical characteristics (Table 1).
Toxicity and feasibility
A total of 1517 cycles of chemotherapy were administered (median 7; range 1–42).One toxic death was reported due to primary tumour perforation followed by haemorrhagic syndrome and septic shock. Bleeding syndrome (digestive or epistaxis)was observed in 12 patients, all treated with antiangiogenic therapies. The most frequent toxicity was a digestive toxicity, grade 3-4 diarrhoea, which occurred in 33 patients (21.6%). Haematological toxicities mainly involved neutropenia. The main toxic events are listed in Table 2. Aflibercept plus the FOLFIRI3 regimen appeared to have increased toxicity compared to the other chemotherapy regimens with both diarrhoea and neutropenia showing increased incidence.
Objective tumour responses and survival
At the time of analysis, 142 patients (93%) had died with a median follow-up of 9.3 mo (range 0.2–40.7 mo).
Considering all patients included in the study, the ORR and DCR were 51% and 62%, respectively. In the irinotecan-experienced group, the ORR and DCR were 46%(13/28) and 64% (18/28), respectively. We then analysed the data according to the treatment regimen in the FOLFIRI3 alone group, we observed an ORR and DCR of 61% and 66%, respectively. In the bevacizumab plus FOLFIRI3 group, we observed an ORR and DCR of 51.5% and 60.5%, respectively. Finally, in the aflibercept plus FOLFIRI3 group, we observed an ORR and DCR of 45% and 64%, respectively. For the entire study population, median PFS and OS were 3.9 mo (95%CI: 3.2-4.9) and 9.4 mo(95%CI: 6.6-12), respectively. Irinotecan-na?ve patients did not show significantly improved PFS or OS with median PFS of 5.2 movs3.7 mo (log-rank testP= 0.15) and median OS of 12 movs9.3 mo (log-rank testP= 0.38). Median PFS and OS were 3.0 mo (95%CI: 0.8-6.9) and 5.6 mo (95%CI: 4.0-20.2), 3.7 mo (95%CI: 3.0-5.3) and 8.5 mo(95%CI: 6.4-10.7), and 4.7 mo (95%CI: 3.3-12.8) and 13.7 mo (95%CI: 7.9-18.7) for FOLFIRI3, FOLFIRI3 plus bevacizumab and FOLFIRI3 plus aflibercept, respectively.Kaplan-Meier curves illustrating PFS and OS in the whole cohort are shown in Figures 1A and B while Figures 1C and D illustrate PFS and OS for the patient subgroups treated with FOLFIRI3, FOLFIRI3 plus bevacizumab and FOLFIRI3 plus aflibercept. The log-rank test shows significantly improved PFS and OS in the FOLFIRI3 plus aflibercept group. Using the Cox univariate model, good performance status, previous surgery of metastases, first through third line therapy and aflibercept usage were associated with better prognosis in terms of PFS. Good performance status and aflibercept usage were also associated with better prognosis in terms of OS (Table 3). Using the Cox multivariate model, only previous surgery of metastasis and aflibercept usage were associated with better prognosis in terms of PFS while good performance status and aflibercept usage were associated with better prognosis in terms of OS (Table 4).
DISCUSSION
This retrospective study is the largest to date reporting FOLFIRI3 regimen efficacy in mCRC. It is also the first study comparing efficacy and safety of the FOLFIRI3 regimen alone or in combination with bevacizumab or aflibercept. This study demonstrates the safety of these three regimens in heavily pre-treated patients with good performance status. The combination of FOLFIRI3 plus aflibercept gives a higher rate of toxic events as a significant number of the patients in this cohort (33%)presented severe diarrhoea in comparison to 11% and 19% in the FOLFIRI3 or FOLFIRI3 plus bevacizumab cohorts. The frequency of severe diarrhoea was alsomore prevalent than what has been previously reported in the VELOUR study, where only 19% of patients had grade 3 or higher diarrhoea[8]. A similar level of diarrhoea was observed in the recent retrospective study of Carola, in which 38% of patients experienced severe diarrhoea events[14]. Our group previously reported the association between severe diarrhoea induced by aflibercept and microscopic colitis[15,16].Aflibercept inhibits placental growth factor (PIGF), which prevents colonic ischaemia and, consequently, induces colitis. In preclinical models, the absence of PIGF promotes dextran sodium sulphate-induced colonic mucosal angiogenesis and increases mucosal hypoxia[17]. Other toxicities such as neutropenia and stomatitis occurred at similar rates across all three chemotherapy regimens[8,18,19]. Few cases of febrile neutropenia were observed probably because 65% of the patients received prophylactic G-CSF treatment. In addition, only 18% of the patients were irinotecanna?ve. Such data may result from selection bias, since only patients who had few prior toxic events while being treated with irinotecan were included and further treated with the FOLFIRI3 regimen.
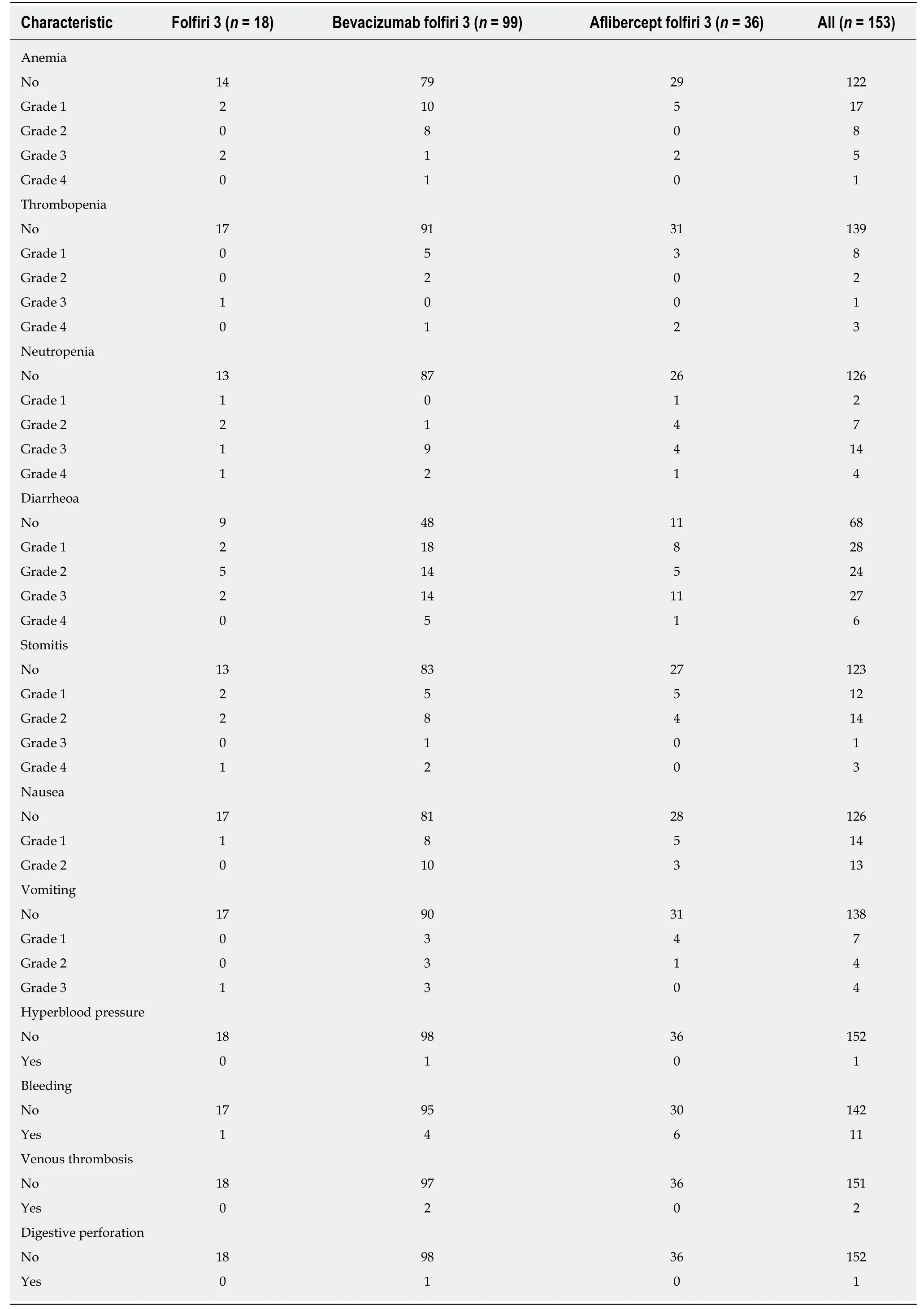
Table 2 Summary of chemotherapy toxicity n (%)

Figure 1 Survival curves for progression free survival and overall survival. A, B: Kaplan-Meier estimates for progression-free survival (A) and overall survival(B); C, D: Kaplan-Meier estimates for progression-free survival (C) and overall survival (D); patients were stratified according to their treatment: FOLFIRI3 (in red),FOLFIRI3 plus bevacizumab (in blue) or FOLFIRI3 plus aflibercept (in yellow). aP-value < 0.05.
Irinotecan hinders DNA replication by inhibiting type I topoisomerase. Inhibition of type I topoisomerase induce single strand DNA breaks. After this initial DNA damage, failure to repair the DNA breaks results in increased apoptosis. Preclinical studies show that the anti-proliferative activity of 5-FU in combination with irinotecan is schedule dependent[20-22]. For example, several studies showed that delayed administration of irinotecan increases FOLFIRI cytotoxicity. Likewise, the FOLFIRI2 regimen (irinotecan delivery post 5-FU injection) induced promising objective responses but suffered from major haematological toxicity[23]. In contrast, FOLFIRI3 has an improved toxicity profile and previous studies showed that this regimen is active in mCRC resistant to FOLFIRI. Furthermore, in the absence of a targeted agent,response rates range from 17 to 23%, with median PFS of 4-7 mo and median OS of 9-12 mo[11,12,24].
In a similar setting, our group previously reported that FOLFIRI3 plus bevacizumab resulted in a 53% response rate and median PFS and OS of 7 and 13 mo,respectively. Importantly, these results did not differ from the FOLFIRI3 results without a targeted therapy[13]. A recent retrospective report on the usage of FOLFIRI3 in combination with aflibercept demonstrated a response rate of 35%[14]. Results were improved in irinotecan-na?ve patients in comparison to the irinotecan-experienced cohort with median PFS and OS of 11.3 mo and 17.0 mo, respectively, for the irinotecan-na?ve group and 5.7 mo and 14.3 mo for the irinotecan-experienced group.Our study mainly involved patients that were previously treated with irinotecan and,in the FOLFIRI3 plus aflibercept cohort, only 5 of the 36 patients were irinotecanna?ve. In this cohort, median PFS and OS were 4.7 mo and 13.7 mo, respectively. This study supports the hypothesis that aflibercept increases the efficacy of the FOLFIRI3 regimen. The main limitation of this study is the retrospective design with a relatively low number of patients per cohort.
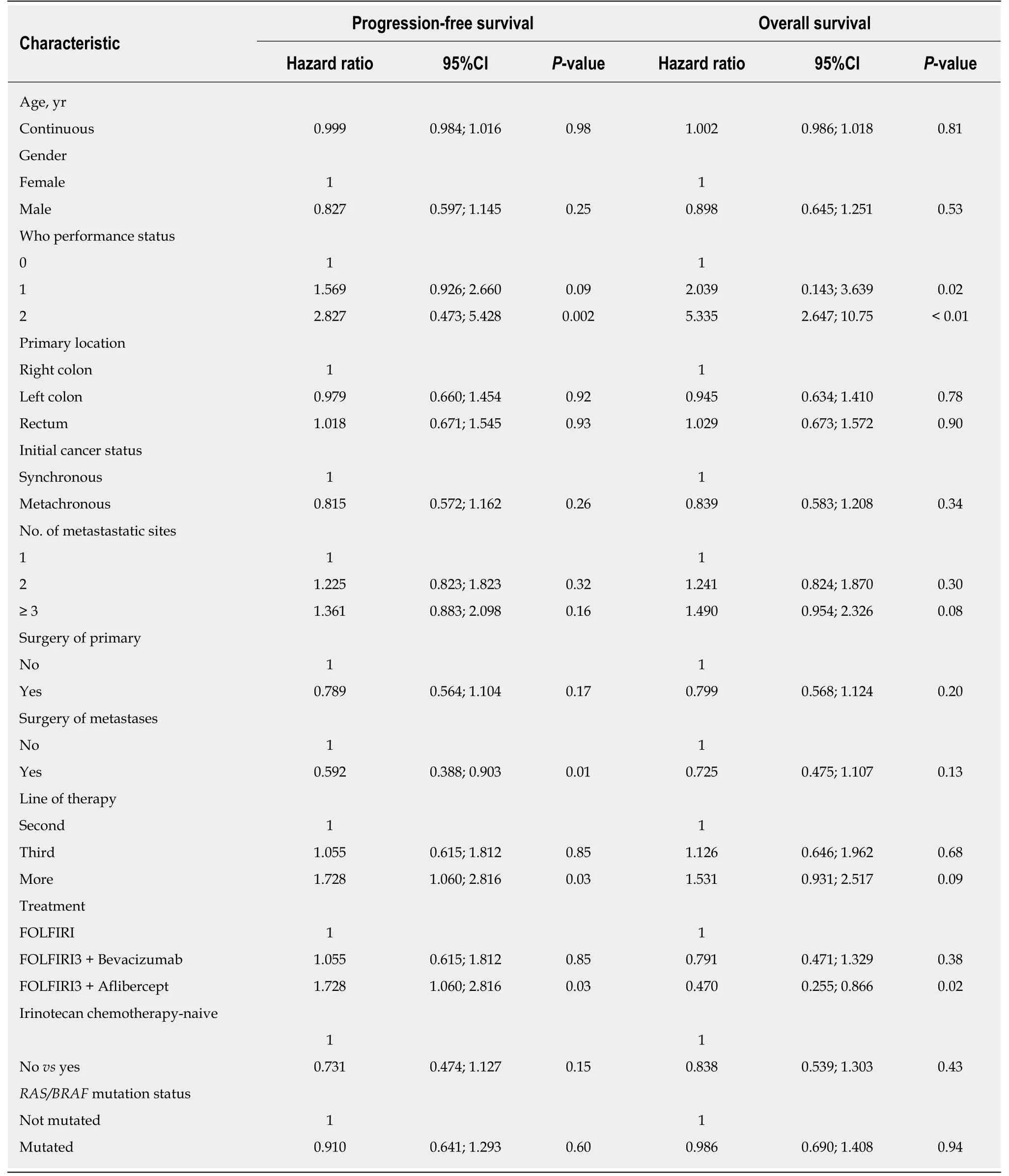
Table 3 Results of Cox univariate analyses
The FOLFIRI3 regimen demonstrates efficacy and safety in patients previously treated with irinotecan and is an alternative strategy for multi-treated patients. The combination of aflibercept and FOLFIRI3 appears more efficacious than FOLFIRI3 alone or in combination with bevacizumab. A randomized trial comparing FOLFIRI3 plus bevacizumabvsFOLFIRI3 plus aflibercept should be conducted to validate this hypothesis.
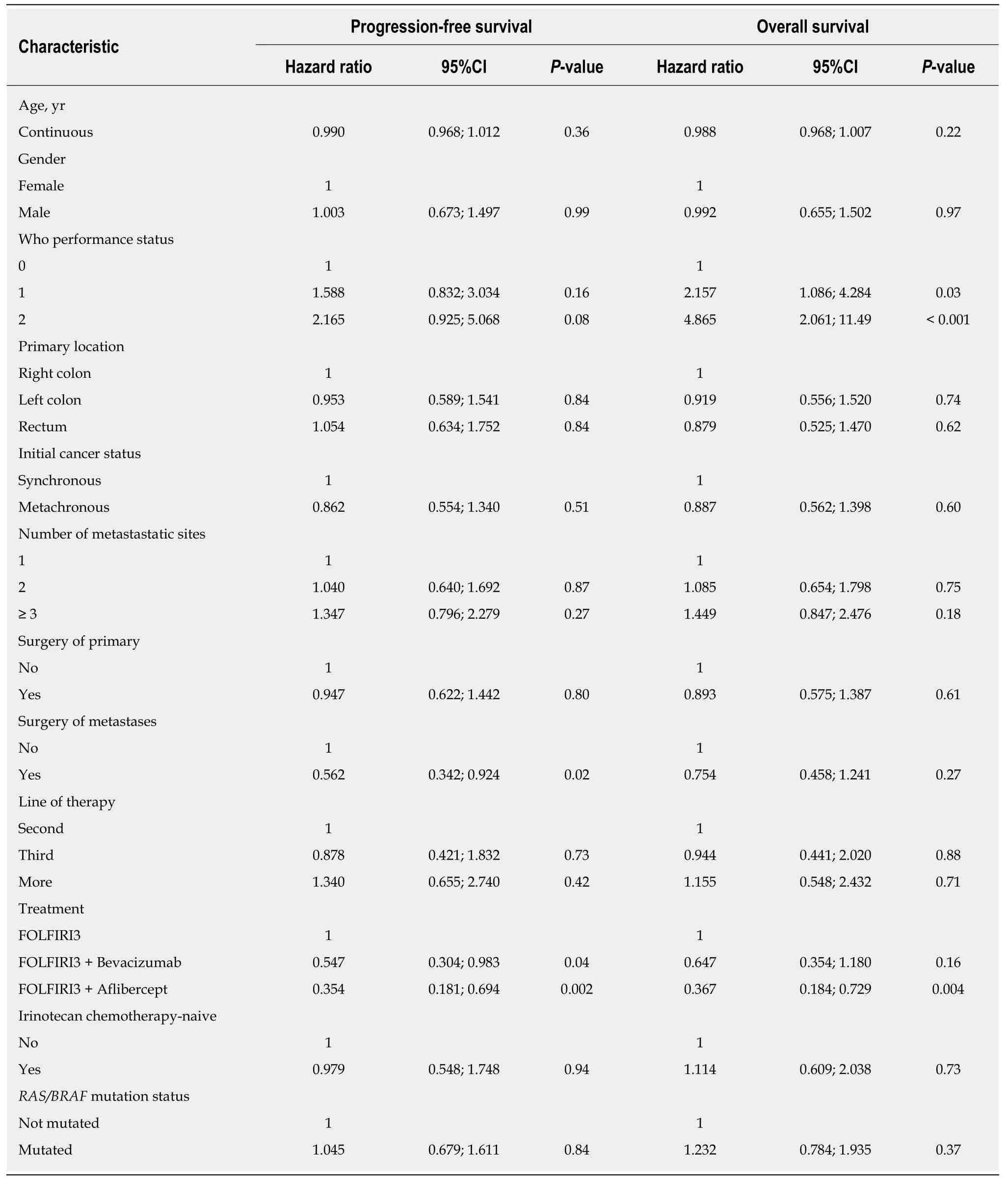
Table 4 Results of Cox univariate analyses
ARTICLE HIGHLIGHTS
Research background
FOLFIRI3 is a modification of the classical FOLFIRI regimen with injection of irinotecan at day 1 and 3. This treatment is used as second or further line in many French Centre’s based of previous retrospective data. This chemotherapeutic regimen could be used alone or in combination with antiangiogenic agent but comparison of efficacy of FOLFIRI3, FOLFIRI3 bevacizumab and FOLFIRI3 aflibercept has never been performed.
Research motivation
Our objective was to compared efficacy and toxicity of FOLFIRI3, FOLFIRI3 bevacizumab and FOLFIRI3 aflibercept regimen.
Research objectives
The main objective of the study is to evaluate the safety and efficacy of the FOLFIRI3-used alone or in combination with bevaicuzmab or aflibercept.
Research methods
This is a monocentric retrospective study evaluating the efficacy and safety of the FOLFIRI3 regimen given alone or in combination with bevacizumab or aflibercept in patients with previously treated metastatic colorectal cancer (mCRC).
Research results
One hundred and fifty-three consecutive patients were included (18 treated with FOLFIRI3, 99 with FOLFIRI3 plus bevacizumab and 36 with FOLFIRI3 plus aflibercept). Median progressionfree survival (PFS) and overall survival (OS) were 3.9 mo (95%CI: 3.2-4.9) and 9.4 mo (95%CI:6.6-12), respectively. Median PFS and OS values were improved in the FOLFIRI3 plus aflibercept group. Grade 3-4 adverse events (diarrhoea and neutropenia) were more frequent in the FOLFIRI3 plus aflibercept group.
Research conclusions
The modification of FOLFIRI regimen had an impacton mCRC patients’ treatment response. The addition of an antiangiogenic agent, in particular aflibercept, enhanced the clinical benefit and improved survival.
Research perspectives
Prospective randomized trial comparing FOLFIRI-aflibercept to FOLFIRI3-aflibercept are warranted.
 World Journal of Clinical Oncology2019年2期
World Journal of Clinical Oncology2019年2期
- World Journal of Clinical Oncology的其它文章
- Challenges in the diagnosis and treatment of gestationaltrophoblastic neoplasia worldwide
- Oligometastases in prostate cancer: Ablative treatment
- Existing anti-angiogenic therapeutic strategies for patients with metastatic colorectal cancer progressing following first-line bevacizumab-based therapy
- Pancreatic cancer screening in patients with presumed branch-duct intraductal papillary mucinous neoplasms
- Impact of conditioning regimen on peripheral blood hematopoietic cell transplant
- Hong Kong female’s breast cancer awareness measure: Crosssectional survey
