Facile synthesis of new selenite Co0.8Ni0.7(OH)SeO3 and its capacitance performance
YIN Haixia, NIU Helin, ZHANG Shengyi
(College of Chemistry and Chemical Engineering, Anhui University, Hefei 230601, China)
Abstract:Aiming to prepare supercapacitor electrode materials, a new selenite Co0.8Ni0.7-(OH)SeO3 was synthesized via a facile chemical approach. The morphology and composition of the products as-obtained were characterized by various test techniques, in detail. In addition, the impedance and electrochemical property of the products were studied by electrochemical impedance spectroscopy and cyclic voltammetry, respectively. Significantly, the charge-discharge test results showed that the Co0.8Ni0.7(OH)SeO3 exhibited high specific capacitance (1 776 F·g-1) and excellent cycling stability, due to the synergistic effect of Co(II), Ni(II) and Se(IV) electroactive components.
Keywords: selenite; cobalt; nickel; synthesis; capacitance
0 Introduction
In the last decades, it has been a research hotspot to develop new energy conversion and storage devices, due to ever-increasing environmental problems and up-coming fossil-fuel depletion. In this field, the energy storage systems with low cost, high efficiency and environment-friendliness, attract much attention and get rapid development. Supercapacitor, as one of important energy storage devices, is most promising power source since it can provide a higher power density than battery and higher energy density than conventional capacitor[1-3]. As it is well known, the electrode material is an important issue in supercapacitors, and the breakthrough for electrode material is full of challenges. In the light of the energy storage mechanism, the supercapacitors can be classified into electrical double-layer (EDL) capacitors, pseudocapacitors and hybrid-capacitors. Usually, the pseudocapacitors whose electrode was made up of the materials-based transition metals, exhibit a higher specific capacitance than EDL capacitors based on carbonaceous materials[4]. Therefore, a series of transition metal compounds (such as oxides, sulfides and selenides) were proposed to supercapacitor materials. For example, various MnO2-based materials have been prepared and used in supercapacitors, due to its abundance, low-cost, nontoxicity, high theoretical specific capacitance and environmental compatibility[5-9].
As a kind of important transition metals, Ni and Co have a lot of advantages, such as good conductivity, high electrochemical activity, environmental friendliness, abundance and low cost. Thus, the materials on the basis of Ni and Co have aroused widespread interest. The oxides such as NiO[10], Co3O4[11]and Ni-Co oxide[12-13]were proposed for supercapacitor materials, the sulfides such as NiS[14], Ni3S2[15], CoS[16], Co3S4[17], Ni1.5Co1.5S4[18]and Co9S8/NiCo2S4[19]were recommended to construct supercapacitor electrodes, and also the selenides such as Co0.85Se[20]and NiSe-CoSe[21]were synthesized and used in supercapacitors.
There are some reports on the syntheses and crystal structures of Ni or Co selenites. However, less work has been reported on the application of Ni or Co selenites. For example, Vlaev and his coworkers[22-23]prepared CoSeO32H2O and NiSeO32H2O crystals respectively by mixing Na2SeO3with Co(NO3)2and NiCl2in aqueous solution and aging the solution at room temperature for a week. Manfred[24]studied the crystal structures of Co3(SeO3)3H2O and Ni3(SeO3)3H2O that were synthesized under hydrothermal conditions (at 210 ℃ for five days), using equimolar amounts of Co(OH)2-KHCO3-H2SeO4and NiO-SeO2-NaOH as precursors, respectively. Miljak and his coworkers[25]discussed the crystal structure and magnetic properties of NiSeO3that was synthesized via chemical vapour transport reaction. Also, Larraaga and his coworkers[26]investigated the thermal and magnetic properties of Co0.4Ni0.6(SeO3)2H2O with specific composition. Recently, CoSeO3compound has been used as cathodic catalyst for oxygen reduction[27]and as electrode material in dye-sensitized solar cells[28], respectively. Here, the Co0.8Ni0.7(OH)SeO3was synthesized by a simple chemical approach, in which Co(NO3)2and Ni(NO3)2were used to react concurrently with Na2SeO3in aqueous solution. Interestingly, charge-discharge test results show that the Co0.8Ni0.7(OH)SeO3has excellent capacitive properties, which made it a promising material in supercapacitor.
1 Experimental section
1.1 Materials and apparatus
CO(NO3)26H2O, Ni(NO3)26H2O, Na2SeO3, K4Fe(CN)6, K3Fe(CN)6, polyvinyl pyrrolidone (PVP), cetyltrimethyl ammonium bromide (CTAB) and sodium dodecyl sulfate (SDS) are all from Chemical Reagent Company. All reagents are of analytical grade and used without further purification. All solutions used in experiments were prepared using deionized water as solvent.
Vacuum drying oven (Bonrun industrial Co. China); centrifugal machine (Changsha common instrument Co. China); scanning electron microscope (SEM, S-4800, Hitachi, Japan); X-ray diffractometer (XRD, XD-3, Purkinje General, China, CuKαradiation,λ=0.154 06 nm, 36 kV, 20 mA); thermogravimetry analysis meter (TG, 449F3, Germany); inductive coupled plasma emission spectrometer (ICP, IRIS Intrepid XSP Ⅱ, China); Fourier transform infrared spectrometer (FTIR, NEXUS-870, Nicolet Instrument Co. USA); laser confocal micro Raman spectrometer (In Via-Reflex, RENISHAW); X-ray photoelectron spectroscopy (XPS, VG Escalab MKII, England); electrochemical workstations (IM6, ZAHNER, Germany; CHI-660D, Shanghai Chen Hua Instrument Co. China).
1.2 Synthesis of Ni-Co selenite
Typically, the Ni-Co selenite was synthesized as follows. First, 0.146 g (0.5 mmol) CO(NO3)26H2O and 0.146 g (0.5 mmol) Ni(NO3)26H2O were dissolved in 40 mL water in a round-bottom flask. Then, under stirring, 20 mL solution containing 0.346 g (2 mmol) Na2SeO3was slowly added in the flask that had been placed in oil-bath at 50 ℃. Finally, after the flask containing reaction solution was kept at 50 ℃ for 1 h, the purple precipitate formed in solution was separated out, and washed with water and ethanol for several times, respectively. The Ni-Co selenite was obtained by drying purple precipitate at 90 ℃ in vacuum oven for a day. For comparison, pure CoSeO3and NiSeO3products were synthesized according to similar approach as above, respectively.
1.3 Characterization and measurements
The products as-synthesized were characterized by XRD, SEM,XPS, FTIR and Raman, respectively. The Nyquist plots of electrochemical impedance spectroscopy (EIS) were measured in 0.01 M PBS (pH=7.4) solution containing 0.002 M Fe(CN)64-/3-and 0.1 M KCl, with three-electrode system: Ag/AgCl (sat. KCl) reference electrode, platinum foil counter electrode and glassy carbon work electrode (3 mm in diameter) modified with the products. For the measurements of electrochemical and capacitive properties, Ag/AgCl (sat. KCl) reference electrode and platinum foil counter electrode were used as above, and the work electrode was prepared as follows. First, the nickel foam (1 cm×3 cm) was immerged in 5% hydrochloric acid with ultrasonic for several minutes, and washed with ethanol for several times and dried in a vacuum oven. Then, the electroactive mixture was prepared by mixing the Ni-Co selenite products, acetylene black and polyfluoroethylene according to a mass ratio of 8∶1∶1, by adding small amount of N-methyl pyrrolidone (NMP) as dispersion medium. Finally, the electroactive mixture was grinded uniformly, and the viscous fluid mixture as-obtained was coated onto the cleaned nickel foam. Before use, the work electrode as-prepared was dried in vacuum at 90 ℃ for 12 h, and was pressed at a pressure of 10 MPa. The electrochemical and charge-discharge tests were performed on electrochemical workstation in 6 M KOH electrolyte solution at room temperature.
2 Results and discussion
2.1 Characterization results
The characterization results of the products are shown in Fig.1.From Fig.1A and 1B, it is seen that the pure CoSeO3product is made up of short-rod aggregate with bulk crystals, and pure NiSeO3product has a flocculent structure. Fig.1C shows that the Ni-Co selenite has granular morphology. Comparing three images in Fig.1, it is concluded that the Ni-Co selenite has relatively uniform structure. As can be seen from Fig.1D, there are few weak diffraction peaks on the XRD patterns of the products. The wide diffraction peaks at 33.56° and 63.63° on the XRD pattern of NiSeO3correspond to (121) and (123) facets of orthorhombic NiSeO3crystal (JCPDS No.31-0913), and a weak diffraction peak at 31.65° on the XRD pattern of CoSeO3resulted from the (223) facet of monoclinic CoSeO3crystal (JCPDS No.47-0903). Clearly, the Ni-Co selenite composite has the diffraction peaks of both NiSeO3and CoSeO3. However, these wide and weak diffraction peaks reflect the poor crystallization of the products.

A: SEM image of CoSeO3; B: SEM image of NiSeO3; C: SEM image of Ni-Co selenite; D: XRD patterns of the products.Fig.1 Characterization results of the products
The elementary composition of the Ni-Co selenite was determined by ICP analysis,with dissolving the sample in dilute nitric acid. According to the ICP results, the atomic ratio of Ni∶Co∶Se is calculated to be 0.8∶0.7∶1.0. Based on this atomic ratio, the chemical formula of the product is deduced to be Co0.8Ni0.7(OH)SeO3. Here, the hydroxy radicals come from the Na2SeO3alkaline solution used in synthesis. In addition, the content of Co is higher than that of Ni in the product, perhaps, since the solubility products (pKs=6.8) of CoSeO3is lower than that (pKs=5.0) of NiSeO3[29].
To evaluate the thermostability of Co0.8Ni0.7(OH)SeO3, the thermoanalysis was carried out and the results are shown in Fig.2.
From thermogravimetry analysis result, as shown on curve (I) in Fig.2A, it is seen that the mass fraction (MF) has a remarkable decrease before 150 ℃. This weight-loss can be assigned to the evaporation of surface adsorption water on the sample. Afterwards, the MF dropped slowly from 150 to 370 ℃. Consulting the reported results in which the Co(OH)2and Ni(OH)2were decomposed to oxides from 150 to 350 ℃[30-31], it is concluded that our weight-loss from 150 to 370 ℃ is assigned to the lost of H2O formed by the decomposition of hydroxy radical. According to curve (I), it is obtained that the weight-loss for decomposition H2O is about 3.7% that is close to that (3.9%) calculated from Co0.8Ni0.7(OH)SeO3. Finally, there are two fast weight-loss courses from 370 to 630 ℃. Referring the literature[22-23], the weight-loss in this temperature range resulted from the volatilization of SeO2that was formed by the decomposition of CoSeO3and NiSeO3. The curve (II) in Fig.2A shows the weight loss process of the sample that had been calcined at 300 ℃ for 1 h before thermoanalysis. From the pattern, it is seen that the sample has no dehydration process, and only a loss process SeO2, with a fast weight-loss from 370 to 630 ℃. Form curve (II), it is obtained that the weight-loss for SeO2is about 45.6% that is slightly lower than that (47.8%) calculated from Co0.8Ni0.7(OH)SeO3, perhaps, since the SeO2was not fully volatilized at 630 ℃.
Fig.2B shows the results of differential scanning calorimetry. Clearly, as shown on curve (I), there is a strong endothermic peak at about 80 ℃ due to dehydration process of the sample. However, if the sample had been calcined before thermoanalysis, the endothermic peak disappeared as shown on curve (II). In addition, the relationship lines (dashed lines in Fig.2B) of temperature and time are identical for the samples before and after calcined.
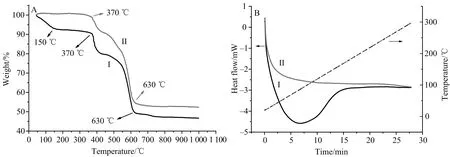
A:Thermogravimetry analysis; B: Differential scanning calorimetry; Curve I: Original sample; Curve II: The sample calcined at 300 ℃ for 1 h.Fig.2 Thermoanalysis results of the Co0.8Ni0.7(OH)SeO3
As it is well-known, the XPS is a technique to examine the chemical situation of elements on the surface of samples[32]. The XPS test results for Co0.8Ni0.7(OH)SeO3are shown in Fig.3 in which the binding energies have been standardized by referencing the value (284.8 eV) of C 1s. Fig.3A shows the high resolution scan of Co 2p peak, in which the Co 2p3/2and 2p1/2peaks with satellite peak can be observed. The binding energies at 781.6 and 797.6 eV are respectively assigned to Co2+2p3/2and Co2+2p1/2, which are in good agreement with the reported values[33-34], meaning that the Co element mainly exists in Co2+state.

A: Co 2p core level spectrum; B: Ni 2p core level spectrum; C: Se 3d core level spectrum; D: O 1s core level spectrum; E: Survey spectrum.Fig.3 XPS spectra of Co0.8Ni0.7(OH)SeO3
Similarly, on the Ni 2p XPS spectrum as shown in Fig.3B, there are two main peaks (2p3/2and 2p1/2) whose binding energies are 856.4 and 873.9 eV respectively, which suggests that the oxidation state for Ni is mainly the Ni2+state[34-35]. Also, there are two weak satellite peaks at the high binding energy side of the Ni 2p3/2and 2p1/2peaks, which means the existence of little Co3+. Fig.3C shows that Se 3d peak locates at 58.5 eV that is similar to the reported value of Se—O bond in SeO32-[36-37], which indicates that the Se element exists as Se4+state. From Fig.3D, it can be seen that O 1s peak is at 531.3 eV that could be assigned to the bond energy of O—Se, rather than Co—Ni—O (529.7 eV) or O—H (532.2 eV)[38-39]. Finally, the XPS survey spectrum in Fig.3E indicates that the selenite contains Co, Ni, Se and O elements.
The products were studied by IR spectroscopy and the absorption spectra are presented in Fig.4A. Clearly, as shown in Fig.4A, there are similar absorption bands on the spectra of three products, which are interpreted by consulting the works reported[22-23, 40]. The absorption bands at about 3 400 and 1630 cm-1are attributed to stretching vibrations and bending vibrations of O—H, respectively. Clearly, compared with that of CoSeO3and NiSeO3products, the absorption bands of Co0.8Ni0.7(OH)-SeO3are weak, which means fewer adsorbed H2O molecules. In addition, the absorption bands at about 700 and 500 cm-1are the characteristic stretching vibrations (Se—O) and bending vibrations (O—Se—O) in SeO32-groups, respectively.

A: FTIR absorption; B: Raman absorption.Fig.4 The spectra of the products
The Raman spectra of the products in the 250 - 1 750 cm-1region are shown in Fig.4B. For three products, the intense absorption band at about 820 cm-1is assigned to the symmetric stretching vibrations of SeO32-radical groups[41]. On the spectrum of NiSeO3product, there is an absorption band at about 430 cm-1which belongs to the bending vibrations of SeO32-. However, for CoSeO3, there is no obvious bending vibrations of SeO32-, which is similar to the results reported in literature[28]. For Co0.8Ni0.7(OH)SeO3, the absorption bands at 820 cm-1and 430 cm-1are weaker than that of NiSeO3, which may result from the introduction of Co(II) to NiSeO3.
2.2 Electrochemical property and charge-discharge performance
As it is known, the EIS analysis is an effective method to evaluate the interfacial electrical conduction of electrode materials. From the point intersecting the real axis in the region of high frequency on the Nyquist plots of the EIS, the internal resistance (Rs) of the electrolyte and electrode can be obtained. According to the semicircle-like part at high frequency region, the electron transfer resistance (Ret) caused by Faradaic reactions and double-layer capacitance can be derived out. Based on the Nyquist plots shown in Fig.5A, the values ofRs/Retare calculated to be 452.3/1 572.4, 720.8/807.7 and 292.8/954.8 (Ω) for CoSeO3, NiSeO3and Co0.8Ni0.7(OH)SeO3, respectively. Obviously, the combination of CoSeO3and NiSeO3enhanced the electric conductivity of the selenite. Cyclic voltammetry (CV) is an effective method to evaluate the electrochemical and capacitive properties of electrode materials. Fig.5B shows the CV curves of the products in 6 M KOH solution. Obviously, there is a pair of redox peaks on the CV curves of three products, revealing pseudocapacitive characteristic. In literature[42-43], it has been reported that in alkaline electrolyte, Co3O4and NiO (or Ni(OH)2) could be respectively oxidized to CoOOH and NiOOH at about 0.3 V vs Ag/AgCl. Therefore, for the products, a pair of redox peaks could be assigned to the electron transformation between oxidation state and reduction state of Co and Ni, and relevant electrochemical reactions are expressed as follows.
CoSeO3+OH-←→CoOOH+SeO2+e-
(1)
NiSeO3+OH-←→NiOOH+SeO2+e-
(2)
In addition, the SeO32-radical in selenite may take part in electrode reaction since its oxidation potential (from SeO32-to SeO42-) is 0.05 V (vs. SHE) in strong alkaline solution[44]. From the CV curves in Fig.4B, it can be concluded that the Co0.8Ni0.7(OH)SeO3has the strongest redox peaks among three products, which suggests its best electrochemical activity.
Fig.5C shows the galvanostatic charge-discharge curves of the products at 1 A·g-1current density. Clearly, the charge-discharge curves of three products are all asymmetric, which is the characteristic of pseudocapacitor. According to the method reported[34], the specific capacitance can be calculated from following equation
(3)
whereI(A) is the discharge current, Δt(s) is the discharge time,m(g) is the mass of the active materials on the electrode, and ΔV(V) is the discharge potential.

A: Nyquist plots of the products; B: CV curves of the products; C: Galvanostatic charge-discharge curves of the products; D: Charge-discharge curves of Co0.8Ni0.7(OH)SeO3 at different current density (insert: capacitance as a function of current density); E: Capacitance of Co0.8Ni0.7(OH)SeO3 as a function of KOH concentration; F: Charge-discharge cycle stability of Co0.8Ni0.7(OH)SeO3.Fig.5 Measurement results
Based on the discharge curves and given current density, the specific capacitances were calculated to be 583, 1 163 and 1 776 A·g-1for CoSeO3, NiSeO3and Co0.8Ni0.7(OH)SeO3, respectively. Obviously, Co0.8Ni0.7(OH)SeO3has the largest specific capacitance among three products. Here, the excellent capacitance performance of the Co0.8Ni0.7(OH)SeO3is due to its good electric conductivity and electrochemical activity. Fig.5D shows the galvanostatic charge-discharge curves of Co0.8Ni0.7-(OH)SeO3at different current densities. From insert in Fig.5D, it is seen that the largest specific capacitance was obtained at 1 A·g-1. The effect of KOH concentration on the specific capacitance of Co0.8Ni0.7(OH)SeO3is shown in Fig.5E, which indicates that the 6 M KOH solution is optimum concentration. Finally, the long-term cycle stability of Co0.8Ni0.7(OH)SeO3at 1 A·g-1was examined by repeating 500 charge-discharge cycles. As shown in Fig.5F, the specific capacitance declined by 15% at first 50 cycles, and afterwards the specific capacitance maintained almost stable until 500 cycles, which suggests that the Co0.8Ni0.7(OH)SeO3has good cycle-stability. Perhaps, the inceptive decline of the specific capacitance resulted from the dissolution of the reaction products (such as SeO2) from electrode surface to solution.
Furthermore, the effect of synthesis conditions on the capacitance of the products was studied and the test results are shown in Fig.6. In typical synthesis,the reaction temperature was set at 50 ℃. If the temperature was decreased or increased, as shown in Fig.6A, the capacitance performance of the product all declined. Fig.6B shows the effect of mole ratios of raw material on the capacitance of the products. From the patterns, it is concluded that the best capacitance of the product was obtained by the mole ratio 1∶1∶4 (as that in typical condition). Looking forward to improving the capacitance of the products, various surfactants were used in synthesis in order to adjust or control the structure of the products. In typical synthesis, no surfactant was added in reaction solution. However, as shown in Fig.6C, whether cationic surfactant CTAB, anionic surfactant SDS or non-ionic surfactant PVP was added in reaction solution, the capacitance performance of as-obtained products declined, instead of increasing.
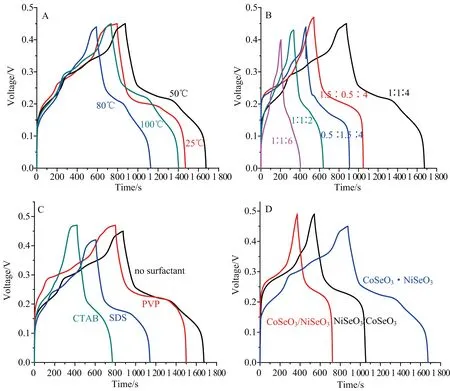
A: Reaction temperature; B: Mole ratios of raw material (Co(NO3)2∶Ni(NO3)2∶Na2SeO3); C: Different surfactants; D: Formation sequence of CoSeO3 and NiSeO3.Fig.6 Effect of synthesis conditions on the capacitance of the products
Finally, the effect of formation sequence of CoSeO3and NiSeO3on the capacitance of the products was investigated. In typical synthesis, 2 mmol Na2SeO3reacted with the mixture of 0.5 mmol Co(NO3)2and 0.5 mmol Ni(NO3)2in solution, which means CoSeO3and NiSeO3were formed almost simultaneously (the product denoted as CoSeO3NiSeO3). When 2 mmol Na2SeO3reacted first with 0.5 mmol Co(NO3)2then with 0.5 mmol Ni(NO3)2, the CoSeO3/NiSeO3product was produced. Similarly, the NiSeO3/CoSeO3product was prepared by reverse reaction sequence. The charge-discharge curves of as-obtained products are shown in Fig.6D, which indicates that the reaction sequence in synthesis has the effect on the capacitance of the products.
For the sake of exploring the effect mechanism on capacitance, the morphology of the products obtained in different conditions was measured, and the results are shown in Fig.7. From the SEM images, it can be concluded that the morphologies of products obtained in various conditions are similar to that of Co0.8Ni0.7(OH)SeO3product obtained in typical condition. In other words, no product with particular morphology was produced. Therefore, the chemical composition and interface-property between particulates may play more important role than the morphology on the capacitive properties of the products.

Fig.7 SEM images of products obtained via (A) reaction at 25 ℃; (B) reaction at 80 ℃; (C) reaction at 100 ℃; (D) in presence of PVP; (E) CoSeO3/NiSeO3 product; (F) NiSeO3/CoSeO3 product
In literature, the capacitance properties of Co-Ni based materials have been studied, and some results are listed in Tab.1. Clearly, comparing with data reported, our Co0.8Ni0.7(OH)SeO3shows best capacitance performance, which is discussed as follows. First, various electroactive ingredients in Co0.8Ni0.7(OH)SeO3, such as Co(II), Ni(II) and Se(IV), cooperated with each other and then increased the redox activity of the electrode. Secondly, as known, Co(OH)2and Ni(OH)2are all good electrode materials in supercapacitor, and therefore, the hydroxy radicals in Co0.8Ni0.7(OH)SeO3are conductive to the capacitance increase. Finally, in fabricating our electrode, the electroactive mixture used to coat nickel foam is viscous fluid that can permeate into the holes of nickel foam, which benefited the electron transfer between electroactive mixture and nickel foam.
Tab.1Specificcapacitance(F·g-1)ofCo-Nibasedcompositesatdifferentcurrentdensities
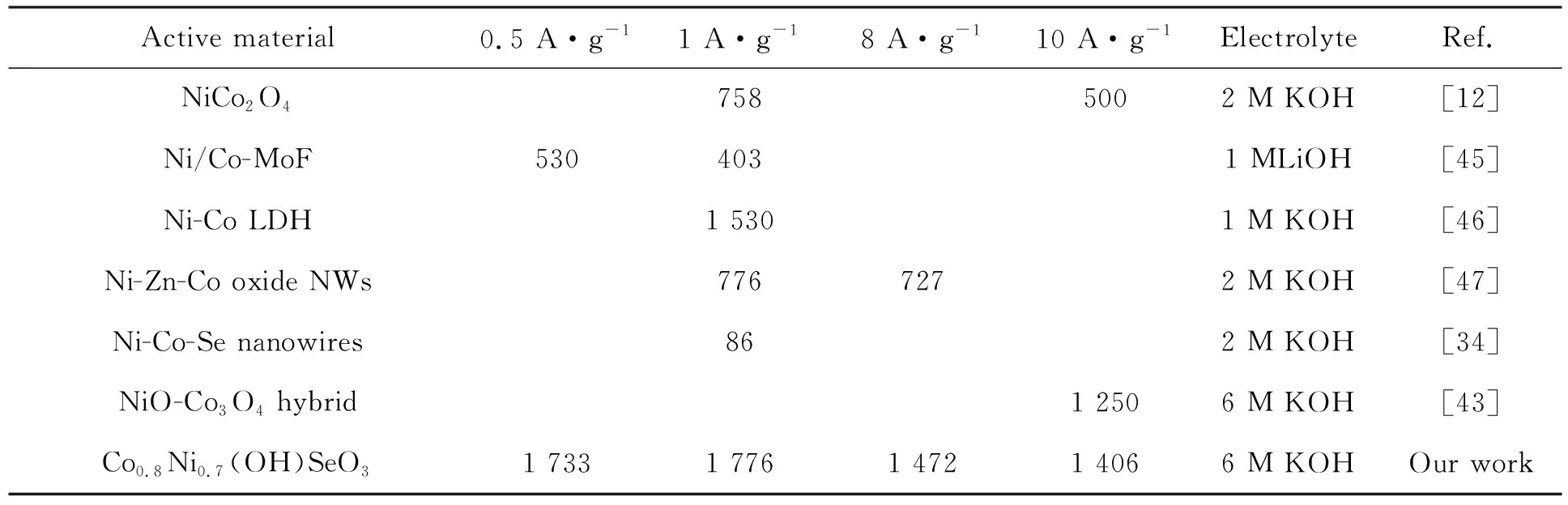
Active material0.5 A·g-11 A·g-18 A·g-110 A·g-1ElectrolyteRef.NiCo2O47585002 M KOH[12]Ni/Co-MoF5304031 MLiOH[45]Ni-Co LDH1 5301 M KOH[46]Ni-Zn-Co oxide NWs7767272 M KOH[47]Ni-Co-Se nanowires862 M KOH[34]NiO-Co3O4 hybrid1 2506 M KOH[43]Co0.8Ni0.7(OH)SeO31 7331 7761 4721 4066 M KOHOur work
3 Conclusions
In summary, a very simple approach for the synthesis of new selenite (Co0.8Ni0.7(OH)SeO3) is proposed, and the products as-obtained were characterized by a series of testing technologies. Based on the test results for the effect factors on the morphology and property of the products, the optimum synthesis condition was set up. Significantly, in virtue of the mutual promotion of multifold electroactive ingredients such as Co(II), Ni(II) and Se(IV), as well as the assistance of OH (I), the Co0.8Ni0.7(OH)SeO3demonstrated high specific capacitance (1 776 F·g-1at 1 A·g-1) and long-term stability. The superior capacitance performance of Co0.8Ni0.7(OH)SeO3to other Ni-Co based materials reported, suggests its potential application in the field of energy storage.

