Gladius growth pattern and increment of jumbo squid(Dosidicus gigas)in the tropical Pacific Ocean
Yi Gong,Yunki Li,b,c,d,e,*,Xinjun Chen,c,d,e,**,Xiodi Go,Ling Chen
aCollege of Marine Sciences,Shanghai Ocean University,999 Huchenghuan Rd.,Shanghai,China
bLaboratory for Marine Fisheries Science and Food Production Processes,Qingdao National Laboratory for Marine Science and Technology,106 Nanjing Rd.,Qingdao,China
cThe Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources,Ministry of Education,999 Huchenghuan Rd.,Shanghai,China
dNational Engineering Research Centre for Oceanic Fisheries,Shanghai Ocean University,999 Huchenghuan Rd.,Shanghai,China
eNational Demonstration Center for Experimental Fisheries Science Education,Shanghai Ocean University,999 Huchenghuan Rd.,Shanghai,China
fCollege of Marine Ecology and Environment,Shanghai Ocean University,999 Huchenghuan Rd.,Shanghai,China
Keywords:Dosidicus gigas Gladius Growth pattern Increment
A B S T R A C T Gladius is an accepted hard tissue for determining the age,size-specific growth,and trophic dynamics of pelagic squid.However,little is known about the inter-and intra-stock variability of gladius growth pattern and increment deposition.In this study,the gladius growth patterns with somatic growth and gonad development were evaluated for two geographic stocks of the jumbo squid(Dosidicus gigas)in the tropical Pacific Ocean.The microstructure and periodicity of the gladius growth increments were also investigated.Results showed varied correlations between four gladius morphometric characteristics with dorsal mantle length,while growth in body weight almost followed a power model.Sexual gladius growth patterns occurred with gonad development,possibly due to different biological functions of these gladius parts and sex-specific energetic allocations.Gladius increments were observed formation in the stem and lateral plate and could be consistently enumerated.The daily periodicity of increment deposition,at least over 144 days old,was validated by comparing gladius increments with the ages obtained from the statoliths.The different oceanographic conditions between two areas likely influence the gladius growth and increment deposition and promote higher increment counts in squid from equatorial waters.Above all,these results provided new information on gladius microstructures and growth increment in different stocks of D.gigas and confirmed the use of this tissue for size-specific growth and age determination.
1.Introduction
Gladius,or pen,is the typically hard tissue of a pelagic squid(Arkhipkin,Bizikov,&Fuchs,2012).It is a chitinous internal structure that lies within the dorsomedial site of the mantle cavity.Compared with other hard tissues(e.g.statolith and beak)of squid,gladius is more reliable to determine the size-specific growth in squid,because the gladius length is highly correlated with mantle length and its growth rates similar to those of longitudinal mantle growth(Jackson,Arkhipkin,Bizikov,&Hanlon,1993;Perez,O'Dor,Beck,&Dawe,1996;Bizikov&Arkhipkin,1997;Schroeder&Perez,2013).From the tail fin towards the head,the gladius consists of three morphological parts:rostrum,conus,and proostracum(Fig.1).Recently,growth increments have been observed along the dorsal surface of the proostracum in squid species such asSepioteuthis Lessoniana(Jackson et al.,1993),Illex illecebrosus(Perez et al.,1996)andI.argentines(Schroeder&Perez,2013).In these species,the growth increments deposition showed daily periodicity during a large part of its life,suggesting the potential applications in squid age and growth rates determination.Moreover,the gladius growth increments are easier to count since they can be read with less pretreatment than statolith method(Arkhipkin&Shcherbich,2012).For example,after washed with warm soapy water,the gladius increments ofS.Lessonianacould directly count using a zoom magnification with light(Jackson et al.,1993).However,the enumeration of increments cannot be reliably estimated by this method,because they become faint in the posterior region of the gladius and early growth is consequently masked(Jackson et al.,1993;Perez et al.,1996).
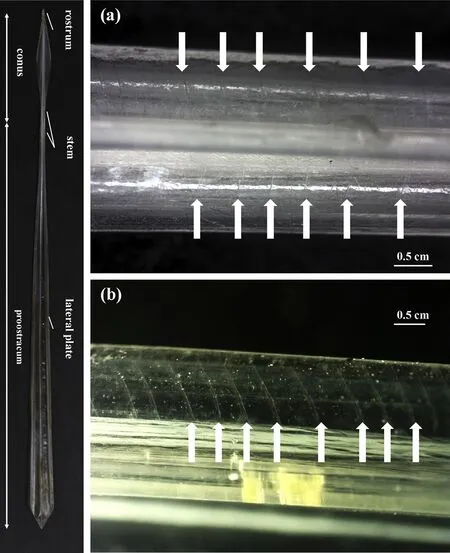
Fig.1.Photographs of gladius of jumbo squid(Dosidicus gigas),a 26.7cm mantle length individual from the waters off Peruvian exclusive economic zone.(a)growth increments on the surface of the stem(marked with white arrows)and(b):growth lines in the edge of the lateral plate.
With the continuous developments of serial sampling of time recording structure for stable isotope analysis,gladius has been considered as an accepted structure for studying high-resolution trophic dynamics in squid species of commercial importance(Kato et al.,2016;Li,Gong,Zhang,&Chen,2017;Rosas-Luis,Navarro,Martínez-Baena,&Sánchez,2017;Ruiz-Cooley,Villa,&Gould,2010).For instance,sequential isotopic signatures along theOmmastrephes bartramiigladii showed different migratory behaviors between females and males(Kato et al.,2016).Using stable isotope analysis of gladius,Ruiz-Cooley et al.(2010)observed ontogenetic shifts in habitat use and diet ofDosidicus gigasin the eastern Pacific Ocean.Li et al.(2017)used a time-based consecutive sampling ofD.gigasgladius to evaluate the influence of historical events occurring in squid lifetime.However,little attention has beenpaid to the variability in gladius growth patterns in squid from different origins with distinct environmental conditions,although the latter may result in a phenotypic divergence in gladius(Gong,Li,Chen,&Fang,2018).In addition,several studies have shown that sexual size dimorphism usually occurs in the hard tissues of squid even for the same geographic stock(Fang,Chen,Su,Thompson,&Chen,2017;Gong et al.,2018).
The jumbo squid,D.gigas,is a pelagic cephalopod endemic to the eastern Pacific Ocean(Nigmatullin,Nesis,&Arkhipkin,2001).This squid supports the major fisheries in the eastern tropical Pacific and the coastal waters of western South America.As a voracious predatorand a source of prey,D.gigascan transfer energy from the mesopelagic food web to higher trophic level marine organisms(Field,Baltz,Phillips,&Walker,2007).Because of the important economic and ecological role ofD.gigas,numerous studies analyzed its foraging strategies,migration patterns and population structure in the past decades(Keyl,Argüelles,&Tafur,2010;Nigmatullin et al.,2001).In the studies mentioned above,D.gigasgladius has already been used to evaluate the trophic dynamics(Li et al.,2017;Ruiz-Cooley et al.,2010),though the knowledge of gladius growth patterns remains limited,leading to difficulty in accurately understanding the information from gladius sections.
In this study,D.gigasfrom two harvest locations in the tropical Pacific Ocean were chosen,as they represented different environment conditions(offshore and nearshore tropics).We aimed to(1)evaluate the potential impacts of somatic growth and gonad development on gladius growth at inter-and intra-stock level,(2)determine the microstructure and periodicity of growth increments formation in gladius.This study contributes new information on gladius microstructures in different stocks ofD.gigasand provides an alternative way to determine the size-specific growth and age in this species.
2.Materials and methods
2.1.Squid sampling and processing
D.gigaswere sampled from commercial jigging vessels that operated in waters off the Peruvian exclusive economic zone(PER)and central equatorial Pacific Ocean(CEP)(Fig.2).Squid were frozen on board and sent to the laboratory for processing.The dorsal mantle length(ML)and body weight(BW)of each specimen were measured.Maturity stage was determined according to Lipi'nski&Underhill.(1995).Statoliths were extracted and prepared for age determination.
In total,510 intact gladii from two harvest locations were extracted and cleaned using distilled water prior to analysis(see Table 1 for detailed sampling information).Five morphometric characteristics of gladius were measured according to Gong et al.(2018).These measurements included gladius length(GL),conus length(CL),maximum width of conus(CW),proostracum length(PL)and maximum width of prostracum(PW)(Fig.1).
2.2.Gladius growth increments
A total of 159 statoliths from two geographic stocks were suitably prepared for age determination.Gladii from the same individuals were washed again after morphometric analysis and immersed in plates with distilled water to avoid dehydration.Gladius was wiped drycarefully when start counting the number of growth increments.The increments were observed directly on the stem and lateral plate using a dissection microscope(40×)with an adjustable fiber optic light source and no special preparation.In the area of the stem,the growth lines were observed on its surface(Fig.1a),while consistent cross-sections are shown in the edge of the lateral plate(Fig.1b).The number of increments was counted from posterior of stem towards the anterior end of the lateral plate until the increments become faint(Fig.1).The number of growth increments was eliminated when the difference between two independent counts exceeded 10%(Regueira,González,&Guerra,2015).Therefore,125 specimens(CEP,n=69;PER,n=56),varying from 222 to 408mm MLs,were used to estimate the periodicity of gladius increment with the number of statolith rings.
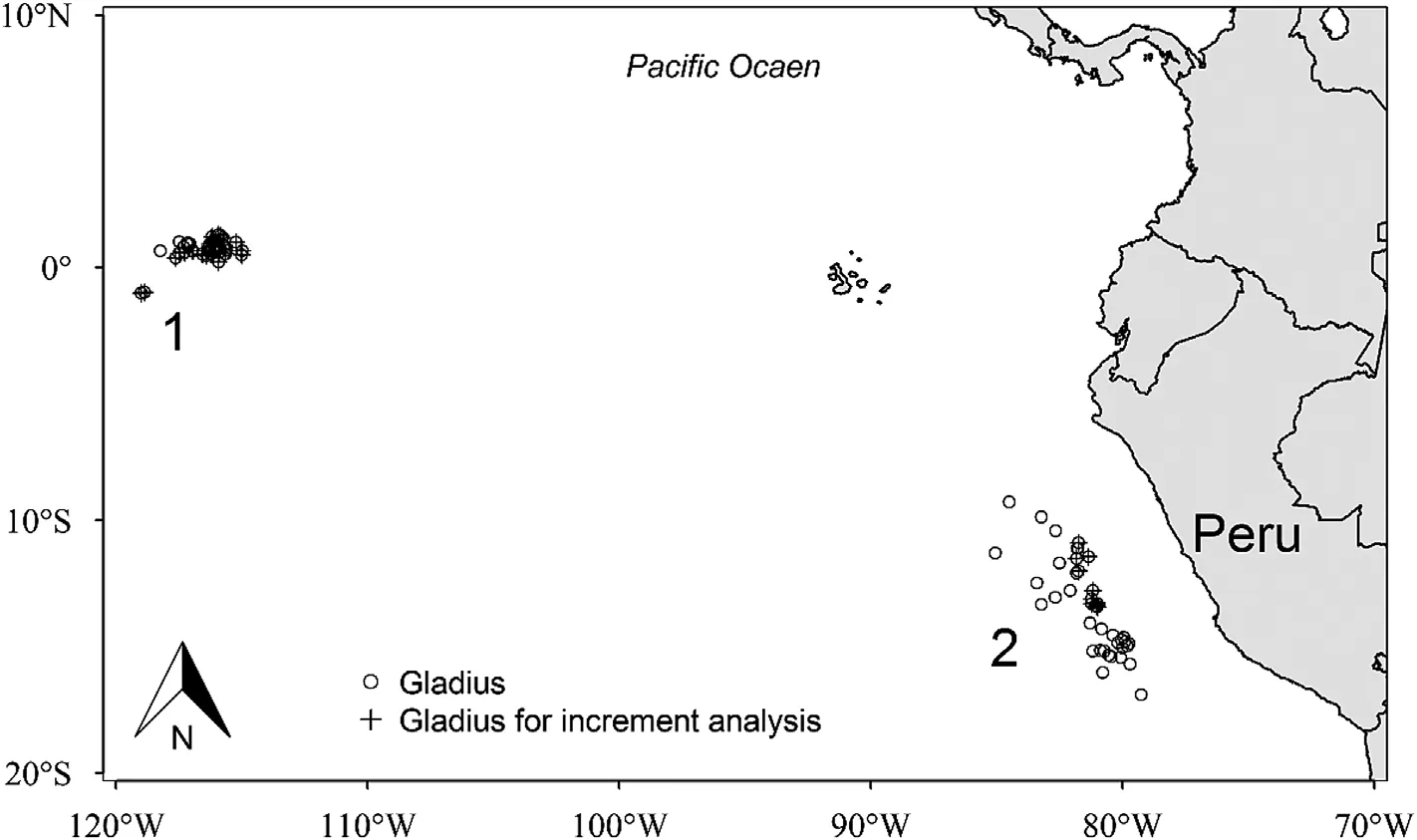
Fig.2.Sampling sites.Dosidicus gigas collected in offshore waters of the central eastern Pacific(CEP,site 1)and off the Peruvian exclusive economic zone(PER,site 2).
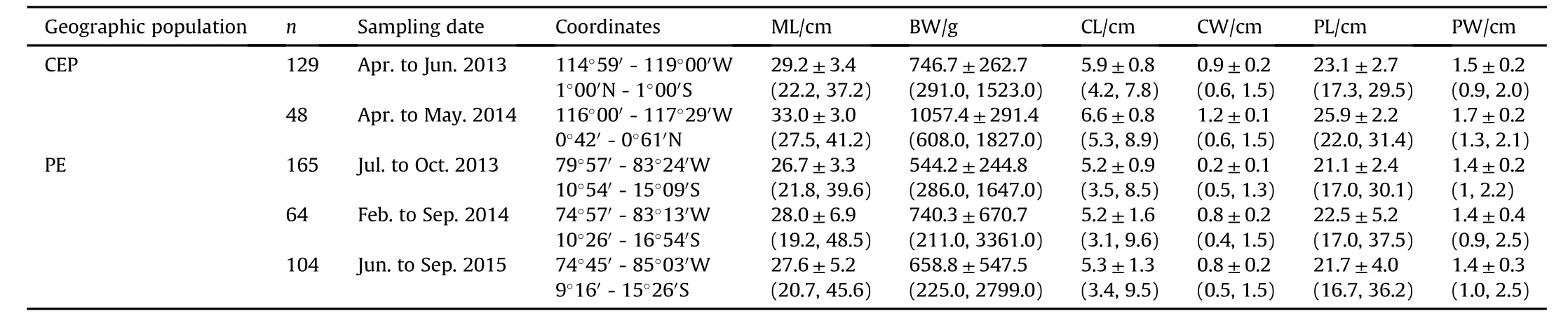
Table 1 Summary information on mantle and gladius of jumbo squid(Dosidicus gigas)sampled in offshore waters of the central eastern Pacific(CEP)and off the Peruvian exclusive economic zone(PER).Values are mean±SD with ranges(minimum,maximum).The measurements are dorsal mantle length(ML),body weight(BW),conus length(CL),maximum width of conus(CW),proostracum length(PL),and maximum width of prostracum(PW).n=number of samples.
2.3.Data analysis
The correlations between squid somatic growth(ML and BW)and gladius microstructure morphometrics(CL,CW,PL,and PW)were explored by fitting linear,power and exponential models,respectively,using the largestr2(coefficient of determination)methods(Table S1).To evaluate the impact of gonad development on gladius growth,one-way analysis of variance(ANOVA)with post-hoc Tukey's honestly significant difference(HSD)test were performed on the variation of gladius morphometrics by different maturity stages.
The relationship between statolith and gladius increment counts was determined using linear regression analysis and using at-test to validate gladius increment deposition rate(Ho:slope=1).
3.Results
3.1.Relationships of somatic to gladius growth
The gladius ofD.gigasis a flexible structure lying along the dorsal midline of its mantle.Strong linear correlations were detected between gladius length and dorsal mantle length in both geographicstocks(CEP:r=0.987,ML=1.031GL-0.644;PER:r=0.990,ML=1.007GL+0.263),while positive power relationships were found with body weight (CEP:r=0.974,BW=0.022GL3.082;PER:r=0.981,BW=0.028GL3.005).
The values of four gladius measurements(CL,CW,PL,and PW)were summarized in Table 1.Analysis ofr2values indicated the best models for ML with CL,CW and PW varied between locations or sexes,respectively(Table 2).In addition,the linear model was suitable for estimating the relationships between ML and PL in both population units.On the contrary,among the three models tested,the power model showed best fit for most correlations between body weights and gladius morphometric variables(Table 2),while the linear relationship was observed between CW and BW in PER squid.
3.2.Gladius growth with gonad development
The maturity stages of specimens examined in this study were estimated to be stages I to V,with the majority(93.3%)being stages I to III.Due to sample sizes might influence the reliability,stages IV and V were excluded from the analysis of evaluating the impact of sexual maturity on gladius morphometric variables.As shown in Fig.3,the values at maturity stages were highly variable among measurements.When comparing morphometric characteristics in each population unit,sex-specific growth patterns occurred among maturity stages.In general,the females had larger values relative to males at the same maturity stage.
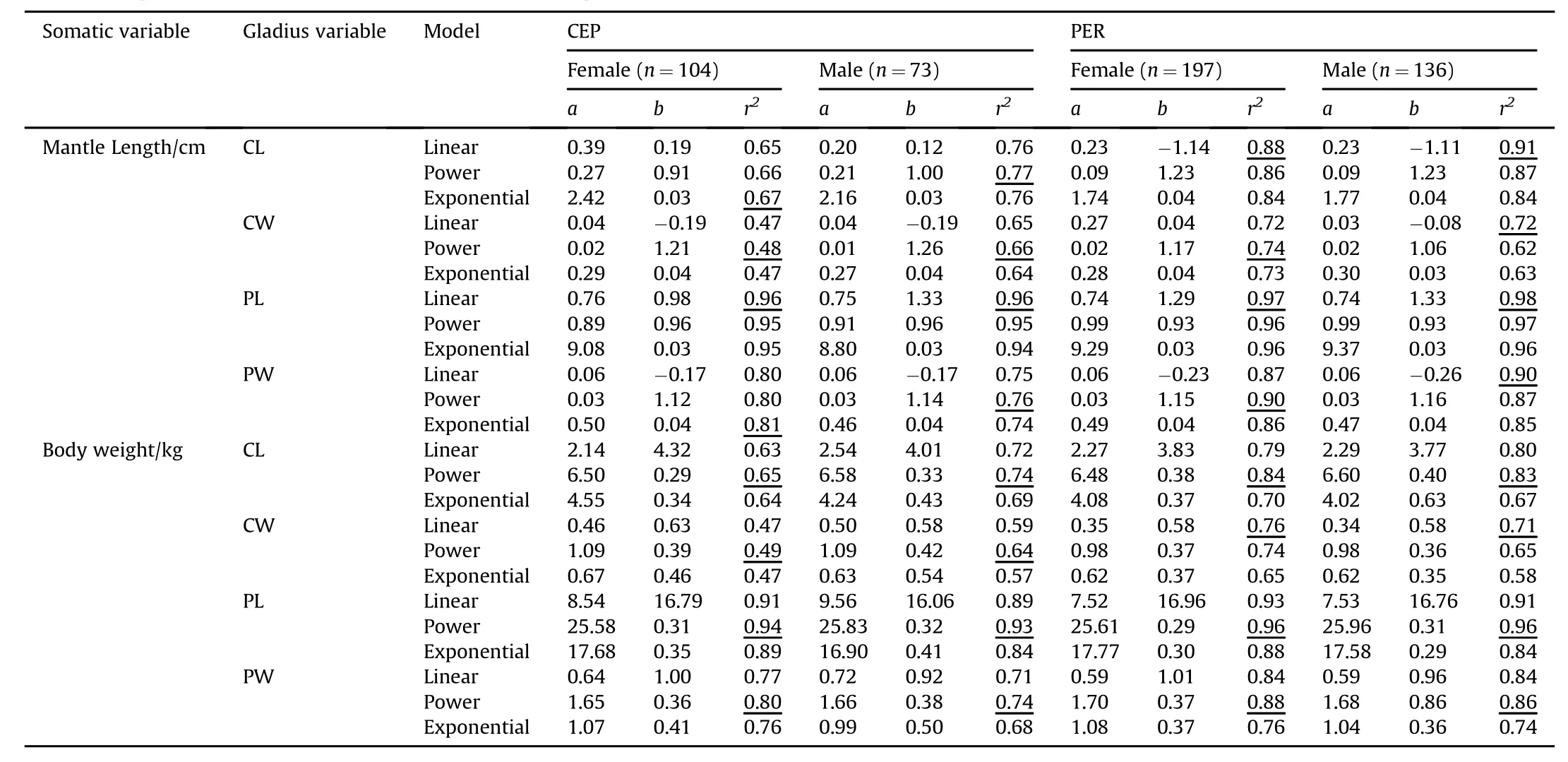
Table 2 List of the selected models and diagnostic statistics for the relationships between gladius morphometric variables and somatic growth of Dosidicus gigas sampled in offshore waters of the central eastern Pacific(CEP)and off the Peruvian exclusive economic zone(PER).The gladius morphometric variables are conus length(CL),maximum width of conus(CW),proostracum length(PL),and maximum width of prostracum(PW).Largest r2(coefficient of determination)were marked with an underline.
3.3.Microstructure and periodicity of gladius increment
On the surface of the stem,gladius increments were defined as thin lines(Fig.1a).In the anterior direction,they progressively became cross sections in the edge of the lateral plate(Fig.1b).The increments were discernible and roughly symmetrically distributed on both sides of the stem and lateral plate edges(Fig.1a),but hardly be recognized at the anterior end of the lateral plate(less than 1/10 of the total length,approximately).A great intraindividual variability of growth rates was showed by distances between consecutive growth increments visualized(Fig.1a and b),although absolute intervals were not measured.
Assuming the increment of statolith is daily deposition(Arkhipkin&Shcherbich,2012),the estimated lifespan of the sampled squid were 141-265 days old.For females,the number of gladius increments showed strong positive relationships with statolith rings(i.e.age in days)in both geographic stocks and the regression slopes were not differing from 1(CEP:t=-0.47,df=30,p>0.05;PER:t=-1.73,df=37,p>0.05)(Fig.4,Table 3).These suggested gladius growth increments were deposited at the same rate as statolith increments,although some increments were faint at the anterior end of the lateral plate.Identical conclusions were obtained in maleD.gigas(CEP:t=1.91,df=35,p>0.05;PER:t=-1.77,df=15,p>0.05).In addition,the count of gladius increments was consistently larger than the number of statolith rings,but their ratios differed among sites and sexes,exhibited lower values in PER squid(mean±SD,1.11±0.10 and 1.18±0.06 for females and males,respectively)than CEP(1.52±0.16 and 1.52±0.13 for females and males,respectively).
4.Discussion
4.1.Gladius growth
The gladius is a flexible internal structure that grows from the tail fin towards the head(Arkhipkin et al.,2012).Strongcorrelations were found for gladius length(GL)versus ML and BW in each population unit,and it confirmed the use of gladius to reconstructD.gigasgrowth patterns.Identical conclusions were obtained inD.gigasfrom the Gulf of California by Ruiz-Cooley et al.(2010)and in other studies of ommastrephids(Bizikov,1995;Kato et al.,2016).Allometric growth is a common phenomenon that occurs in the hard tissues of ommastrephids(Fang et al.,2015).Our results also showed that most of the correlations between the four gladius morphometrics(CL,CW,PL,and PW)and ML were allometric(Table 2).In contrast,the linear model best describes the relationships between PL and ML in both origins and sexes,and it might be considered synchrony between the longitudinal growth of mantle and proostracum.The results possibly due to different biological functions between conus and proostracum,the latter contributes a larger proportion of GL(>80%)and is the main structure to support the internal organs and the lengthening of the mantle(Gong et al.,2018).In addition,sex-specific relationships were occurred at cohort level,for example,the exponential and power models showed best fit for CL with ML of CEP females and males,respectively.Gong et al.(2018)have reported on sexual size dimorphism inD.gigasgladius,which was estimated by using discriminant analysis.This difference was attributed to sexual growth patterns resulting from the divergent energetic allocation for growth or reproduction between sexes(Ferreri,2014;Gong et al.,2018).However,these growth performances were not observed in correlations between body weights with gladius morphometrics,we found that only the relationship between CW and BW in PER squid was not fit power model.Our study also indicated ontogenetic gladius growth patterns associated with changes in maturity stages(Fig.3).In addition,the growth of female gladii was faster than males at the same maturity stage,although different size composition of each sex may have influenced the reliability of calculating values of gladius measurements in some maturity stage.Considering the different size and age at first maturity between female and maleD.gigasin each harvest location(Liu,Chen,Li,&Fang,2017;Liu et al.,2013),the sexual gladius growth patterns with maturity stages may result from the males had an early period in gonad development.
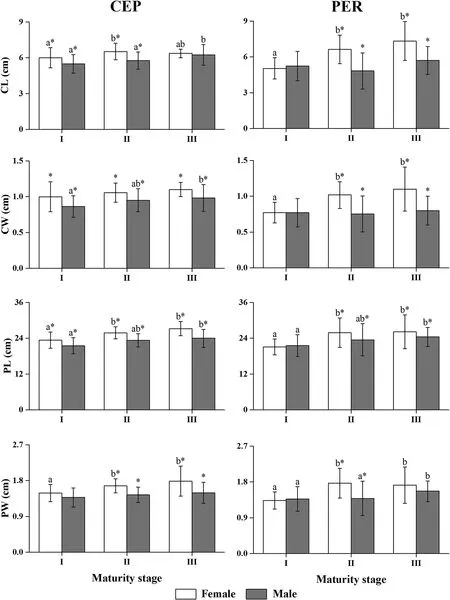
Fig.3.Sex-specific mean values of gladius morphometric variables at different maturity stages. The vertical bars correspond to ±1SD.Dosidicusgigascollected off Peru(PER)and in offshore waters of the central eastern Pacific(CEP).For each sex,alphabetic characters(a,b)above each plot summarize pairwise post-hoc comparisons among maturity stages after analysis of variance(maturity stages not sharing letters were statistically different).Measurements in each maturity stage followed by “*”represents the significant differences between sexes.
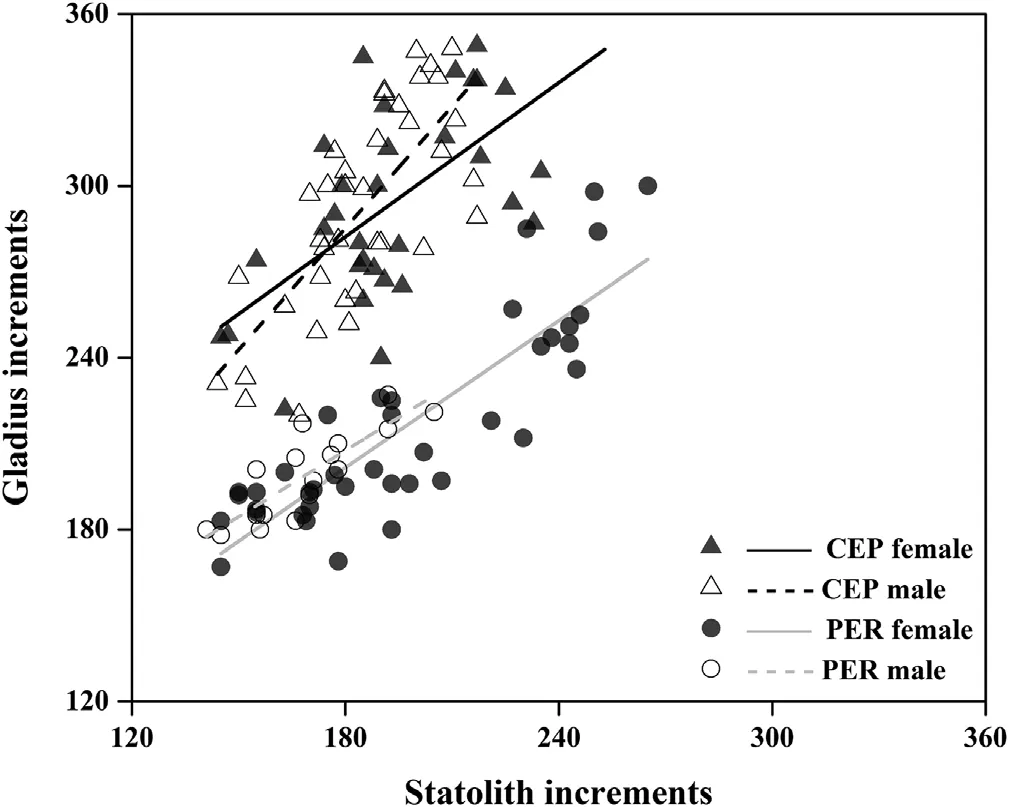
Fig.4.The relationship between the number of Dosidicus gigas gladius increments and statolith increments(age in days).The sex-specific linear regressions were fitted to each geographic population:CEP,offshore waters of the central eastern Pacific;PER,off the Peruvian exclusive economic zone(EEZ).Lines represent significant relationships.See Table 3 for the details of regression equations.
4.2.Growth increment interpretation
It is generally accepted that gladius growth increments along the dorsal surface can be used toage and intra-specific growth rates determination,although the increments cannot be read in the posterior faint zone(Bizikov,1995;Jackson et al.,1993;Perez et al.,1996;Schroeder&Perez,2013).Unlike the previous studies,growth increments were directly observed formation in other gladius microstructures(i.e.stem andlateral plate,Fig.1).It appears that the counting of growth increments are probably more reliable since they can be consistently enumerated in most region of stem and lateral plate.
The correlations between statolith and gladius increment counts in this study validated the daily periodicity of increment deposition in different stocks and both sexes,from 144 to 253(CEP)and 145 to 265(PER)days old(Fig.4),which represents the dominant ages(~70-90%)ofD.gigasin these two areas(Liu et al.,2013,2017).Similar validations were observed in increments along the gladius dorsal surface with statolith rings inS.Lessoniana(Jackson et al.,1993),I.illecebrosus(Perez et al.,1996)andI.argentinus(Schroeder&Perez,2013).They also found that the gladius had a much lower increment counts than that of the statolith rings.On the contrary,the enumeration of gladius increments in this study was at least over 11%as compared with that of statolith rings(Table 3).This difference is thought to depend on different recognizable region and(or)increment formation rates between gladius microstructures in the young squid.Also,there may be an ontogenetic shift in the periodicity of deposition,coinciding with that obtained by Jackson et al.(1993),found that the frequency of gladius increments is more than one per day in young squid,but showed daily deposition when squid over 3 months.Thus,inD.gigasparalarval and(or)juvenile phases,increments appear to be more than one per day but becoming daily periodicity over 144 days old.We assume that this ontogenetic pattern can be substantiated and resolved in the further investigation of youngD.gigas.Another interesting finding is that the CEP squid had a much higher gladius increment counts than that of PER squid at the same age(Fig.4),which may be attributed to the ambient conditions(e.g.temperature)between these two areas(Perales-Raya,Nande,Roura,Bartolomé,Gestal,&et al,2017).The waters off Peru are relatively cooler than CEP since they are primarily influenced by the cold Humboldt Current and upwelling of cold subsurface waters(Anderson & Rodhouse,2001).The lower temperature may consequently decrease the gladius increment deposition rates in the young PER squid(Perales-Raya et al.,2017).
5.Conclusion
Our study identified theD.gigasgladius growth patterns associated with somatic growth and gonad development in two geographic stocks and validated the periodicity of growth increment formation in gladius microstructures.At inter-and intrapopulation level,different growth models were found to fit the correlations between ML and BW with gladius morphometric characteristics.Gonad development also showed significant influence on gladius growth.We suggest that this phenomenon could be driven by different biological functions among gladius microstructures and sex-specific energetic allocations.Growth increments formation in the stem and lateral plate,at least over 144 days old,were a daily deposition.The spatial variation in oceanographic conditions(i.e.temperature)may influence increment deposition.However,given the high plasticity inD.gigasgrowth in relation to environmental conditions and its opportunistic feeding behavior.It is important to identify the temporal variability and whether the observed spatial variations in growth patterns is prevalent across its whole lifespan.Above all,these results confirmed the use of gladius to reconstructD.gigasgrowth patterns,we expect that the novel growth increment counting method has potential applications in squid age and growth rates determination.
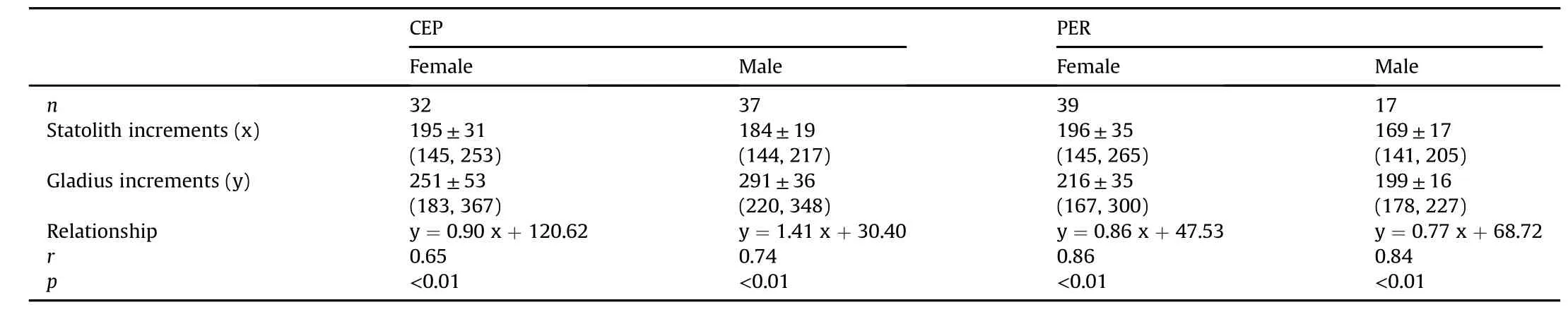
Table 3 Regression equations for the relationships between Dosidicus gigas statolith and gladius increments.Values are mean±SD with ranges(minimum,maximum).CEP,offshore waters of the central eastern Pacific;PER,off the Peruvian exclusive economic zone(EEZ).n=number of samples.na=data not available.
Acknowledgements
We thank observers who helped us with the collection of samples.We also thank Zhang H and Wang S for processing and analyzing of samples.This work was supported by National Natural Science Foundation of China(41541042),National Natural Science Foundation of Shanghai(17ZR1413000),Shanghai Leading Academic Discipline Project(Fisheries Discipline)and Laboratory for Marine Fisheries Science and Food Production Processes,Qingdao National Laboratory for Marine Science and Technology(2017-1A03).
Appendix A.Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.aaf.2018.06.006.
 Aquaculture and Fisheries2018年4期
Aquaculture and Fisheries2018年4期
- Aquaculture and Fisheries的其它文章
- Book review:Aquaculture in China:Success stories and modern trends
- Dermestes maculatus Degeer infestation impact on market loss of dried fish in Kwara State,Nigeria
- Antibacterial mechanism of Ginkgo biloba leaf extract when applied to Shewanella putrefaciens and Saprophytic staphylococcus
- Prey quality impact on the feeding behavior and lipid composition of winter flounder(Pseudopleuronectes americanus)larvae
- Male zebra fish(Danio rerio)odorants attract females and induce spawning
