Light shading improves the yield and quality of seed in oil-seed peony (Paeonia ostii Feng Dan)
HAN Chen-jing, WANG Qi, ZHANG Hong-bao, WANG Shou-hai, , SONG Hua-dong, HAO Jian-mei,DONG He-zhong
1 Cotton Research Center, Shandong Academy of Agricultural Sciences, Jinan 250100, P.R.China
2 Chengwu Agricultural Bureau, Heze 274200, P.R.China
3 Shuangquan Town Extension Station of Agro-Technology, Jinan 250303, P.R.China
1. Introduction
Tree peony is a perennial deciduous shrub with excellent ornamental and medicinal values. It is indigenous to China and has a history of cultivation for more than 2 000 years(Hong and Pan 1999). Tree peony seeds, which are usually a by-product of its flower, have long been neglected, yet recently have been used as an important source of woody oil (Shi et al. 2014). Oilseed peony refers to the tree peony that produces considerable seeds containing a high content of edible oil (Li 2014). By the end of 2015, the cultivated area under oilseed peony in China was 33.3×103ha.Therefore, studies into tree peony are no longer limited to its ornamental and medicinal values; its oil value which is dependent on yield and quality of seeds has become a new focus of research (Han et al. 2015).
The seed yield of crops is mainly determined by the accumulation and distribution of photosynthetic products after anthesis. Generally, tree peony exhibits temporary dormancy under high light intensity and temperature in summer (He 2005). Leaf prematurely appears senescent, withered, curled, or scorched, thereby decreasing photosynthesis and thus, the accumulation of photosynthetic products (Zhou et al. 2008). Appropriate shading significantly increased the biomass accumulation of seedlings and flower bud differentiation in ornamental varieties of tree peony (Zheng and He 2006). Occasionally,the tree peony is grown in non-closed forests to reduce temporary dormancy with such natural shading in northern China. However, it is unclear if appropriate shading would exactly reduce high light intensity stress and increase seed yield in tree peony.
The economic value of oilseed peony is also dependent on seed composition. It is generally believed that seed composition, including fatty acid concentration is affected by environmental conditions and cultural practices.Shading of soybean increased seed protein concentration but decreased seed oil concentration (Proulx and Naeve 2009) while row spacing significantly changed the content of fatty acids (Boydak et al. 2002; Bellaloui et al. 2015).The oil content of castor seeds decreased after shading treatment (Zhang and Liu 2016). The unsaturated fatty acids (UFAs) content of edible oil is an important index for evaluating its nutritional value (Luzzi and James 2001).Tree peony seed oil is unique for its high (>90%) content of UFAs, especially α-linolenic acid (Sevim et al. 2013). The effects of shading on the composition of tree peony seed are poorly understood.
Ornamental tree peony leaves are prone to early senescence under high light intensity and temperature in summer. We hypothesized that appropriate shading delays leaf senescence and thus improves photosynthetic production as well as yield and quality of seed in oil-seed peony. Our objectives were to determine: (i) the effects of shading on leaf senescence in relation to photosynthetic production; (ii) the effects of shading on seed yield and quality parameters; and (iii) most importantly, the appropriate shading regime for seed yield and quality formation in oilseed peony.
2. Materials and methods
2.1. Experimental site and oilseed peony cultivar
The field experiment was conducted in an oilseed peony production base in Shuangquan town (36.35°N, 116.72°E,111 m a.s.l.), Jinan, Shandong Province, China, in 2015 and 2016. The soil at the experimental site was sandy loam containing 0.83% organic matter, 88.5 mg kg–1alkali N, 53.4 mg kg–1available P, and 57.8 mg kg–1available K with a pH of 6.7.
Oilseed peony, Paeonia ostii ‘Feng Dan’, an officially approved (Ministry of Health of China 2011) and most widely cultivated variety in China (Li 2014), was used in the experiment. The oilseed peony trees were two-yearold seedlings transplanted in 2013. Row length×row spacing was 40 cm×60 cm, and the average plant height before shading was 47.5 cm in 2015 and 60 cm in 2016,respectively. Field management practices were conducted according to local agronomic recommendations.
2.2. Shading treatment and experimental design
The trees were covered with different densities polyethylene nets to form light, moderate, severe, and non-shading(CK) treatments. The treatments were arranged into a randomized complete block design with three replications.The area of polyethylene net was 8 m×4 m, and each polyethylene net was supported to as high as 1.4 m by six galvanized steels and held by six anchors. The number of oilseed peony trees was 140 per treatment. Shading started at four weeks after peak flowering and ended at seed harvest.
2.3. Data collection
Photosynthetic photon flux density (PPFD) and air temperature under different density polyethylene nets were measured biweekly after shading. The PPFD was measured with a Li-1400 Photometric System (Li-Cor, USA) at 11:00 a.m.on the cloudless days. Air temperature was measured using mercury thermometers at 12:00 a.m. which were hung between the polyethylene net and the plant canopy.
At four weeks before harvest (late season before maturity), sampled fresh leaves and seeds in partial peonies were frozen in liquid nitrogen immediately and then stored at–80°C for use in analyzing the composition and physiological parameters. The follicles were harvested when fruit peels appeared crab yellow and slightly cracked. Follicles were hand-collected on 11 August, 2015 and 10 August, 2016,respectively. Data were collected for leaf photosynthesis,some shading-related physiological parameters, yield components, and nutritional composition. .
Physiological measurementsPhysiological parameters were determined on five randomly selected plants per treatment at four weeks before harvest. Net photosynthetic rate (Pn) of fully expanded young leaves under different density polyethylene nets was determined with a LI-6400 Portable Photosynthesis System (Li-Cor, USA) at 12:00 a.m.on the cloudless days. For the determination of the maximum net photosynthetic rate (Pmax), leaves were induced for at least 30 min under saturated light intensity before determination. The light intensity was controlled by 6400-02B LED red and blue light sources, and the PPFD was set to 2 000, 1 500, 1 000, 800, 600, 400, 200, 100,and 0 μmol m–2s–1. During the determination, leaves were exposed to the fixed light intensity for at least 200 s, under atmospheric CO2. Photosynthetic light response curve was drawn with PPFD on the horizontal axis and Pnon the vertical axis. The Pmax, apparent quantum efficiency (AQE), light compensation point (LCP), and dark respiration rate (Rd)were calculated with a photosynthetic statistical software.
Leaf chlorophyll (Chl) was extracted with ethanol-acetone(1:2, v/v). Total Chl was fully extracted when leaves were completely white, centrifuged and supernatant collected.Absorbance of the supernatant at 645 and 663 nm for Chl a and Chl b was measured with a spectrophotometer (TAS-990, Beijing, China) with extracted solvent as the blank.
The peroxidation of leaf lipids as indicated by the accumulation of malondialdehyde (MDA) was determined with MDA Assay Kit (TBA method, Nanjing Jiancheng Bioengineering Institute, China). The concentration of proline (Pro) was determined by Pro Assay Kit(Nanjing Jiancheng Bioengineering Institute, China). The concentration of endogenous hydrogen peroxide (H2O2)was measured using an Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen, Carlsbad, CA, USA)according to the method of Zhang et al. (2016). Oxygenscavenging enzyme activities of catalase (CAT), peroxidise(POD) and superoxide dismutase (SOD) were determined following procedures in the manufacture’s guidelines of the respective assay kits (Nanjing Jiancheng Bioengineering Institute, China). The extraction and analysis of endogenous abscisic acid (ABA), auxin (IAA), and gibberellic acid(GA3) were conducted according the procedure described in Zhang et al. (2016). Anti-IgG polyclonal antibody,anti-ABA monoclonal antibody, ABA-conjugated alkaline phosphatase, anti-IAA monoclonal antibody, IAA-conjugated alkaline phosphatase, anti-GA3monoclonal antibody, and GA3-conjugated alkaline phosphatase were all provided by Prof. Wang Baomin of China Agricultural University,Beijing. The alkaline phosphatase activity was visualized with p-nitrophenylposphate as a substrate in 1 mol L–1diethanolamine and 0.5 mmol L–1MgCl2(pH 9.8).Absorbance of the developed color was measured at 405 nm, and the concentration of ABA, IAA, and GA3was calculated.
Yield componentsAt maturity, the number and weight of follicles (excluding shrunken follicles) on all plants under the polyethylene net were determined. Seeds were removed from all follicles and weighted after follicles completely cracked, and the hundred-seed weight and seed yield were determined.
Nutritional compositionThe nutritional composition of seeds - mainly soluble sugars, starch, soluble protein and fatty acid were determined. Soluble sugar and starch were determined by anthrone colorimetric method as described in Ad?o and Glória (2005) and Xue et al. (2015) with minor modifications as follows: About 0.5 g of seeds was milled in a mortar with 10 mL of 80% ethanol. The milled sample was then incubated in boiling water for 30 min and the resulting solution centrifuged at 3 000 r min–1for 10 min. The supernatant was collected for determination of soluble sugar and the pellet was re-dissolved in 10 mL distilled water and 2 mL of 52% perchloric acid for the determination of starch.The soluble sugar and starch were measured at 630 and 485 nm, and calibration curves were plotted with standard solutions of sucrose and potato starch.
Soluble protein was determined by Coomassie brilliant blue G-250 staining method. Seeds (0.50 g) were transferred to a pre-cooled mortar and ground into homogenate with 10 mL distilled water that had been kept on ice. The homogenate was then centrifuged at 3 000 r min–1and 4°C for 20 min. The supernatant was collected for determination of soluble protein. The absorbance was measured at 595 nm and the calibration curve was plotted with standard solutions of bovine serum albumin.
Lipid extraction was conducted as described in Li et al.(2015b) with a slight change. Seeds (0.50 g) were pulverized in a mortar and then transferred to a centrifuge tube with 10 mL n-hexane. After ultrasound-assisted extraction,the samples were centrifuged, and the supernatant was collected. The n-hexane was removed under vacuum at low temperature. Samples were then dried and weighed to estimate the content of crude fat. The crude fat was calculated by using the following formula: Y=(M1–M2)/M(mg g–1)
Where, Y was the content of crude fat (mg g–1); M was the weight of peony seed (g); M1was the total weight of the receiving bottle and sample (oil) (mg); and M2was the weight of the receiving bottle (mg). Fatty acid methylation,GC-MS analysis, and qualitative and quantitative analysis of fatty acids were performed following procedures described in Li et al. (2015b). The content of each fatty acid can be obtained by the results of GC-MS, and then the content of unsaturated fatty acids (mainly the sum of the content of α-linolenic acid, linolein acid, oleic acid)was calculated.
2.4. Statistical analysis
Data were statistically analyzed with SPSS 18.0 (IBM, USA).Means were separated using Duncan’s multiple range tests at P=5%.
3. Results
3.1. Changes in micro-environment
Shading significantly affected PPFD and air temperature as shown in Tables 1 and 2. Averaged across the two years and time intervals, the PPFD was reduced under light shading by 18.4%, moderate shading by 58.6%, and severe shading by 82.1%, being appropriately consistent with the expected reductions in light intensity under the shading treatments.Temperature reductions under light, moderate, and severe shadings were 5.5, 6.2, and 6.4%, respectively.
3.2. Leaf photosynthesis
In the late season (before maturity), shading significantly affected Pnin leaves (Table 3). Pnunder light shading increased by 16.8% but decreased by 14 and 40% under moderate and severe shadings.
The Pmax, apparent quantum efficiency (AQE), dark respiration rate (Rd), and light compensation point (LCP)were fitted to a photosynthetic light response curve (Fig. 1).Shading had significant effects on Pmax, AQE, Rd, and LCP(Table 3). On average, light, moderate, and severe shadings increased Pmaxby 81.4, 58.9, and 27.8%, AQE by 45.7, 34.3,and 17.1%, Rdby 61.5, 44.9, and 41%, and LCP by 43.7,25, and 12.5%, respectively.
Concentrations of Chl a, Chl b, total Chl, and Chl a/b were also significantly affected by shading treatments (Table 4).Averaged accross the two years, light, moderate and severe shading increased concentrations of Chl a by 54.5, 67, and80.7%, respectively; Chl b by 58.8, 82.3, and 105.9%, and total Chl by 52.8, 69.9, and 87%. Severe shading decreased Chl a/b by 11.8%, while other shading treatments did not significantly change the Chl a/b.
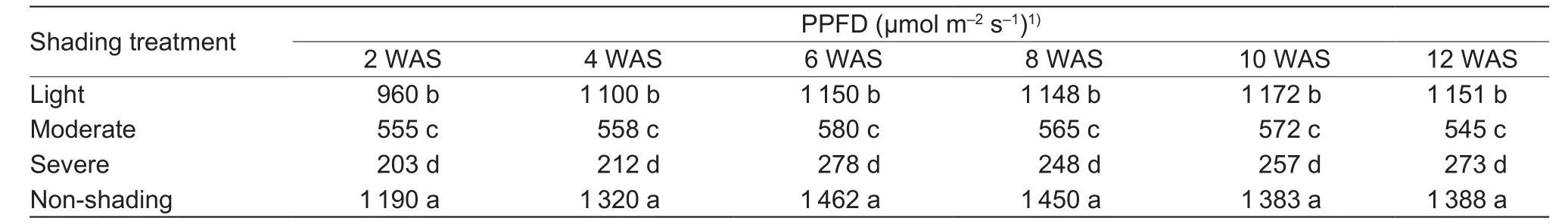
Table 1 Photosynthetic photon flux density (PPFD) at two week internals under different polyethylene nets averaged for 2015 and 2016
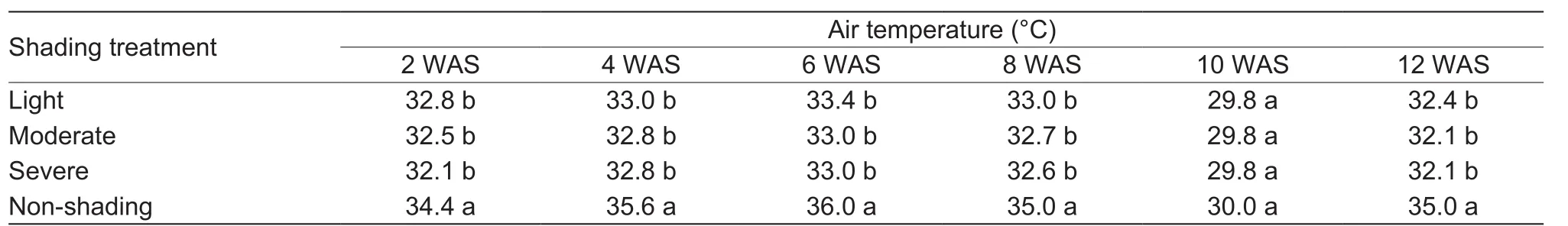
Table 2 Air temperature under different density polyethylene nets every two weeks after shading as averaged for 2015 and 20161)
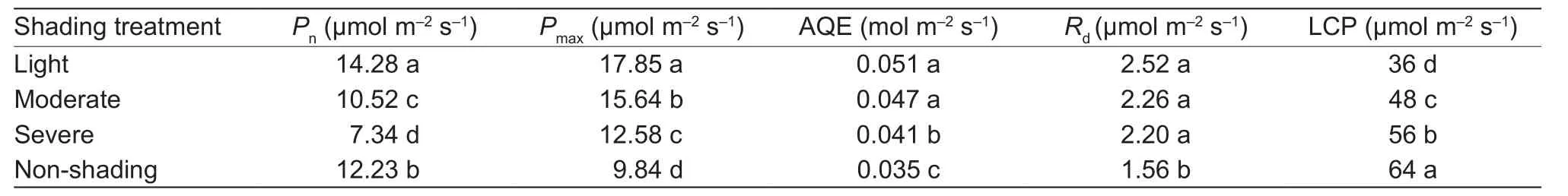
Table 3 Effect of shading on net photosynthetic rate under different shading and the maximum net photosynthetic rate (Pmax),apparent quantum efficiency (AQE), dark respiration rate (Rd), and light compensation point (LCP) of oilseed peony averaged for 2015 and 2016
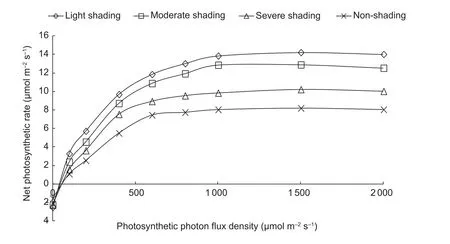
Fig. 1 Net photosynthetic rate of oilseed peony under varying photosynthetic photon flux densities and shading regimes averaged for 2015 and 2016.
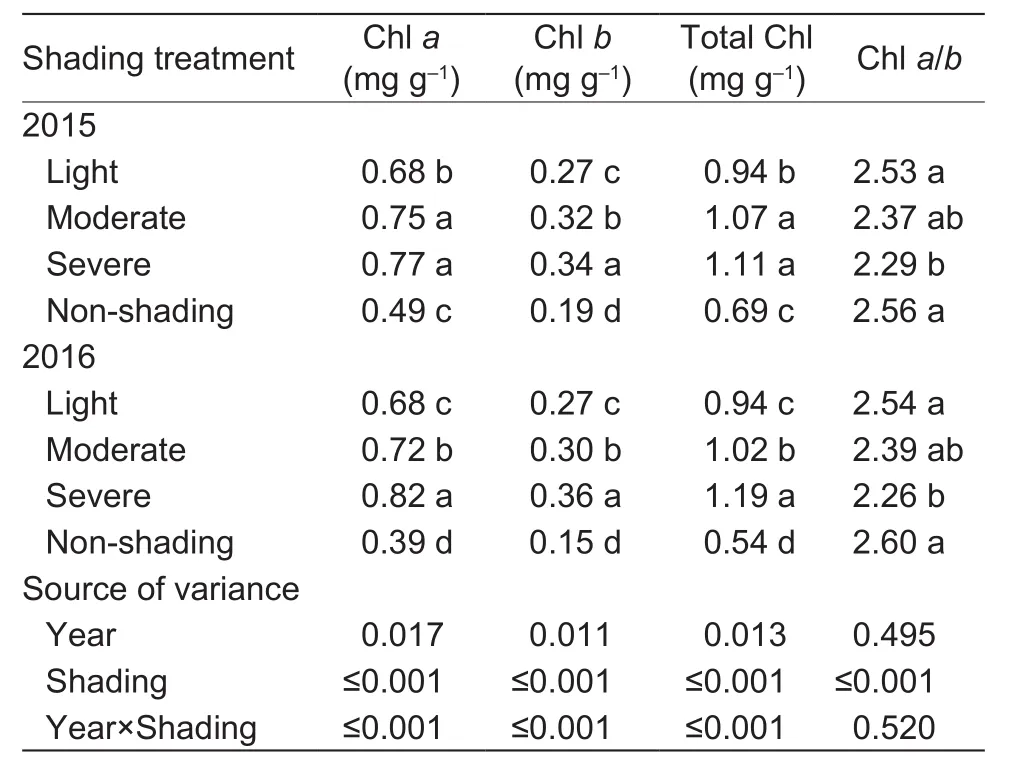
Table 4 Effect of shading on chlorophyll content of oilseed peony
3.3. Lipid peroxidation
Light shading on average reduced the concentrations of H2O2, MDA, and Pro by 24.8, 22, and 18.9%, increased the activities of SOD and CAT by 7.7 and 8.3%, but had no effect on POD (Table 5). In contrast, severe shading did not reduce the accumulation of H2O2or MDA, while moderate shading slightly reduced the accumulation of MDA and not H2O2.
3.4. Endogenous hormones
Leaf concentrations of ABA, IAA, and GA3under the various shading treatments are shown in Table 6. Averaged across the two years, the light, moderate, and severe shadings decreased the ABA concentration by 8.8, 14.4, and 22.7%but increased the IAA concentration by 38.1, 45.5, and 49.0% and GA3concentration by 6.3, 7.6, and 11.7%.
3.5. Yield and yield components
Shading significantly affected seed yield of oil-seed peony in both years (Table 7). Averaged across the two years, light shading increased seed yield by 9.7%, while moderate and severe shading decreased seed yield by 9.8 and 33.3%,respectively.
Yield components, especially the number of follicles per unit land area (follicle density) and hundred-seed weight were significantly affected by shading, while the number of seeds per follicle was little affected (Table 7). On average,light shading increased the seed weight by 7.7%, but had no effect on the follicle density or number of seeds per follicle;moderate and severe shading reduced follicle density by 8 and 25.9%, and severe shading even reduced the seed weight by 8.0%,while moderate shading had little effect.
3.6. Nutritional composition of seed
Shading considerably affected the contents of soluble sugar,starch, soluble protein, crude fat, and UFAs in peony seeds,and these effects varied with year except for starch content(Table 8). In 2015, light shading increased the contents ofsoluble sugar, crude fat, and UFAs by 4.4, 4.9, and 8.4%,but starch and soluble protein contents were comparable to those under non-shading control. In 2016, light shading increased the contents of crude fat and UFAs by 6.2 and 10.9%, but decreased the soluble protein by 21.0%. In 2015, moderate and severe shading increased the content of soluble sugar by 25.0 and 37.8%, but decreased the content of starch by 27.4 and 52.5%, soluble protein by 23 and 44.5%, crude fat by 4.8 and 7.7%, and UFAs by 3.2 and 6.3%, respectively. In 2016, moderate and severe shading increased the soluble sugar content by 24.6 and 56.5%, but decreased the starch content by 20.6 and 49.1%,and soluble protein by 35.4 and 54.9%. Light shading had positive effects on the accumulation of soluble sugar,crude fat, and unsaturated fatty acid. Averaged across the two years, the contents of soluble sugar, crude fat, and unsaturated fatty acid were increased by 6.2, 5.6, and 9.6%,respectively, under light shading.
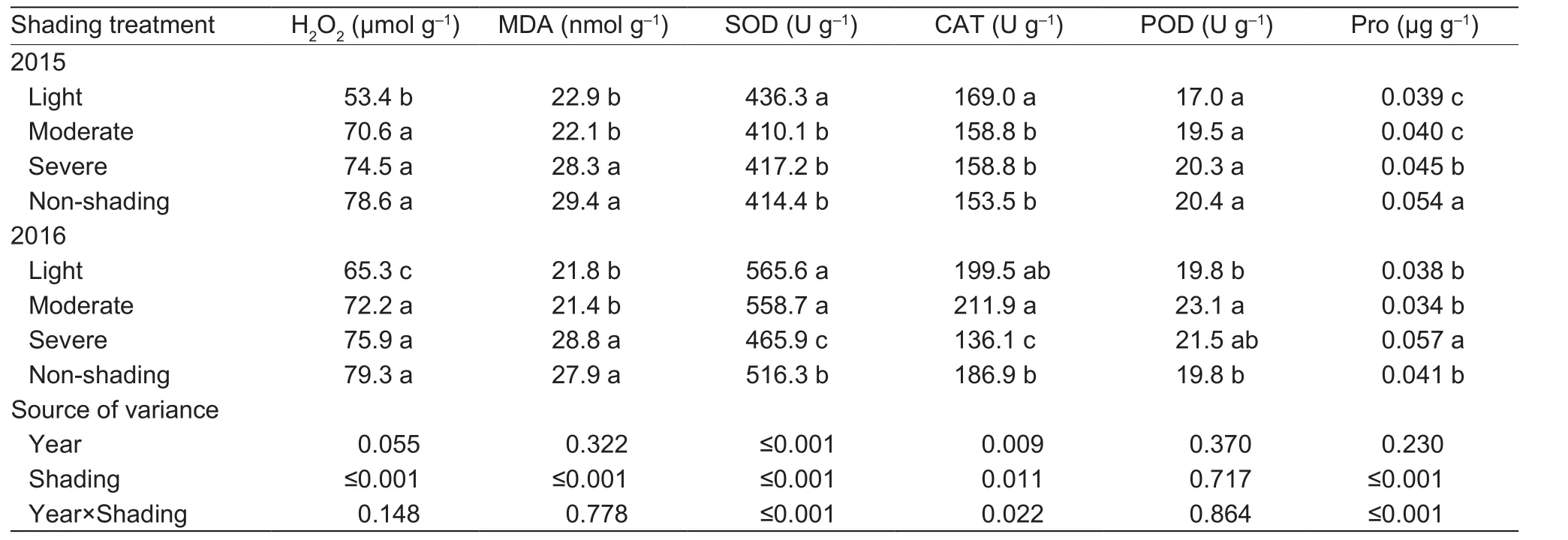
Table 5 Effect of shading on H2O2, malondialdehyde (MDA), antioxidant enzymes activities (SOD, superoxide dismutase; CAT,catalase; POD, peroxidise), and proline (Pro) accumulation of oilseed peony
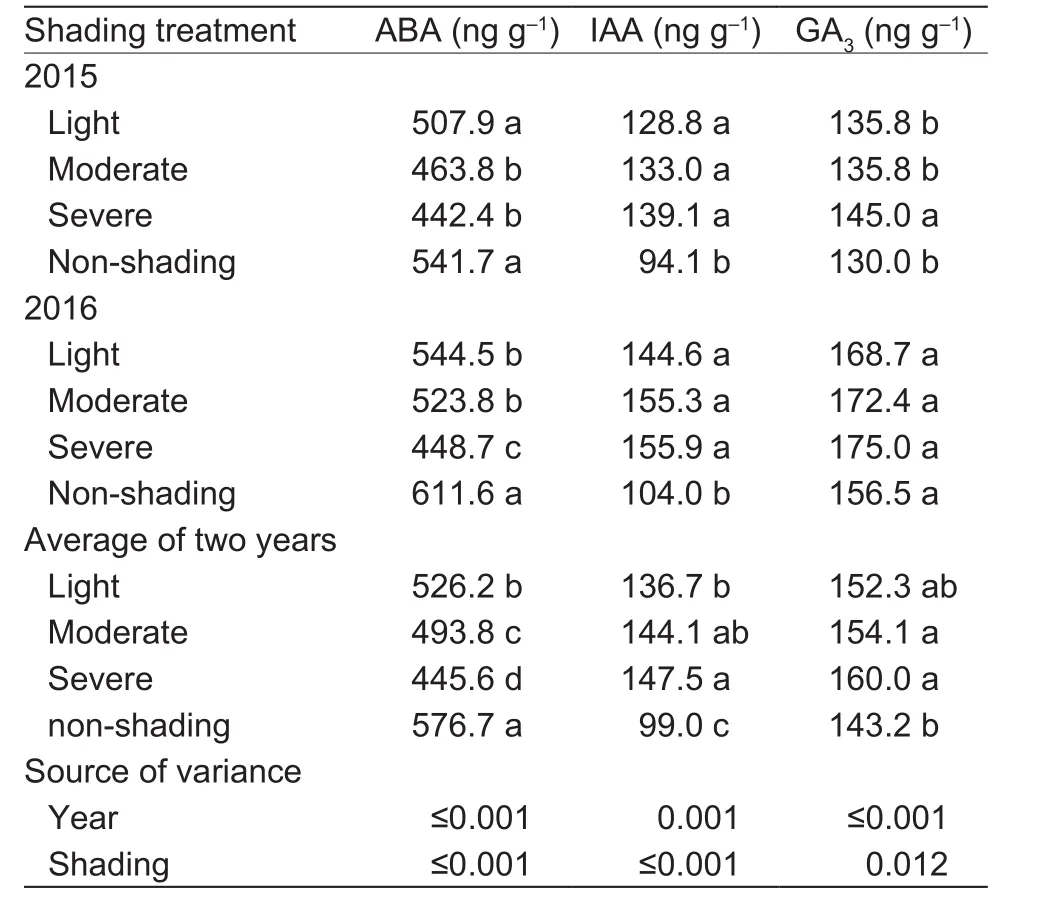
Table 6 Effect of shading on abscisic acid (ABA), auxin (IAA),and gibberellic acid (GA3) concentrations of oilseed peony
4. Discussion
This study has provided a new insight into the common perception that shading affects plant growth and yield in a number of crops. It indicated that light shading delayed leaf senescence and increased photosynthetic production,and then improved seed yield and quality, while moderate or severe shading had significant opposite effects.
4.1. Greater seed weight was possibly due to the delayed leaf senescence and increased photosynthetic production under light shading
Previous studies have shown that shading changes the micro environment which affects photosynetic production,plant growth, and yield formation (Gindaba and Wand 2005),and appropriate shading improved the growth of ornamental peony varieties (Zheng and He 2006; Zhou et al. 2010; Zhu et al. 2012). In the present study, shading not only reduced the light intensity, but also reduced air temperatures as reported previously (Bell and Danneberger 1999; Gindaba and Wand 2005). These changes in micro-climate also resulted in a number of physiological and biochemical changes, which finally affected plant growth and yield. In this study, direct growth indexes of oilseed peony were not measured, but an analysis of endogenous hormones showed that the accumulation of ABA decreased, while the concentrations of IAA and GA3increased. Since ABA is acommon indicator of senescence (Dong et al. 2008, 2009;Chen and Dong 2016), and IAA and GA3are indicators of growth promotion (Zhu et al. 1998), these changes in endogenous hormones indirectly showed that shading promoted plant growth, especially vegetative growth.
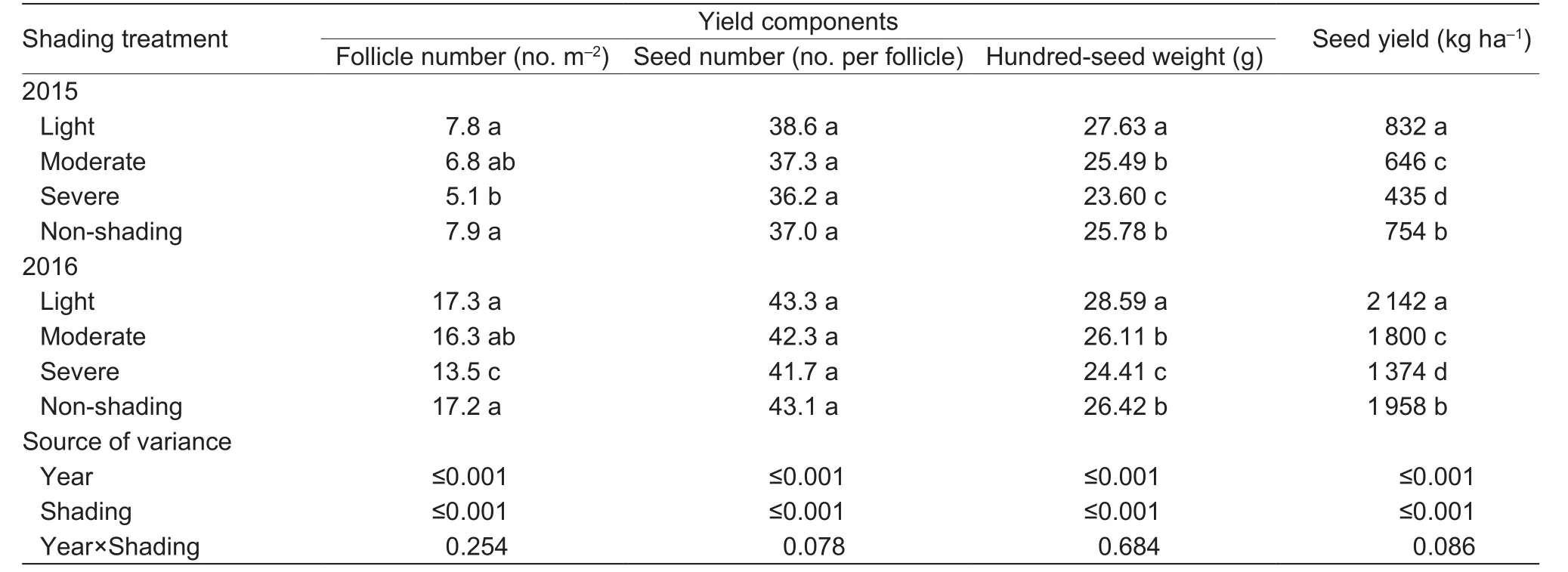
Table 7 Effects of shading on yield and yield components of oilseed peony
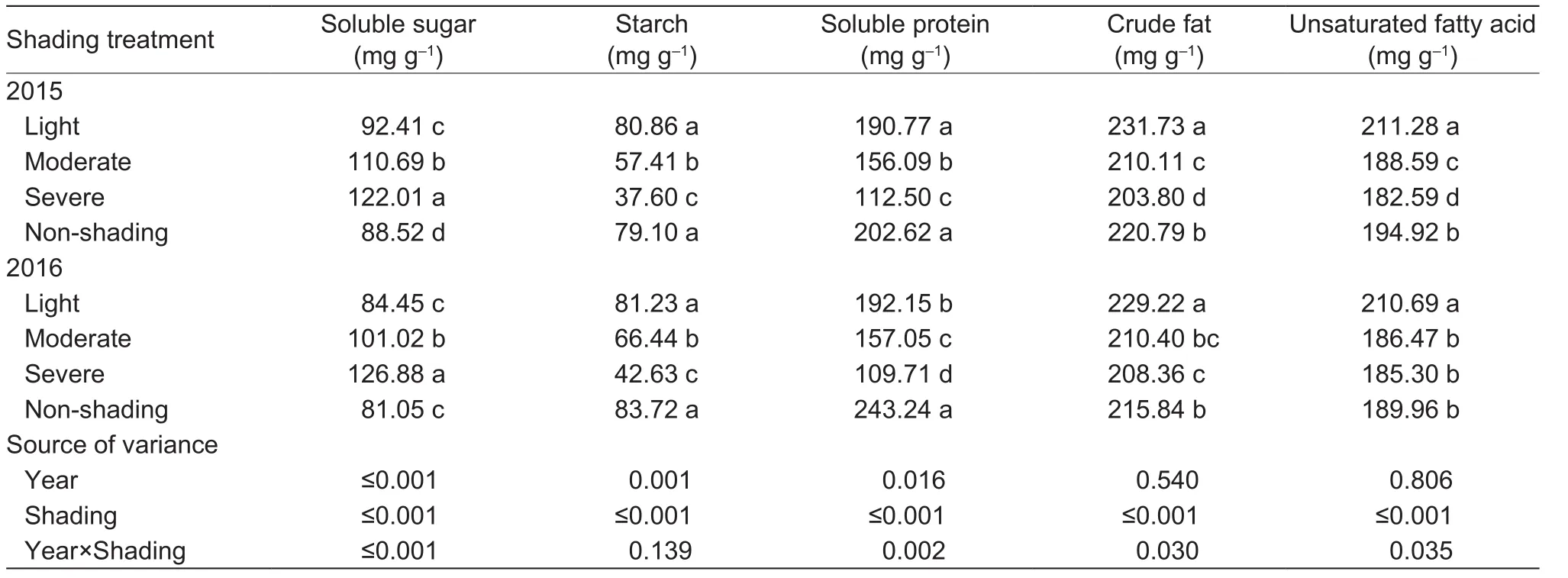
Table 8 Effects of shading on the nutritional composition of oilseed peony
Leaf is the main organ for photosynthesis. With advances in the growth season, oilseed peony trees accumulated a large quantity of H2O2in leaves, which led to leaf senescence as indicated by MDA accumulation and decreased Chl content and net photosynthetic rate. Light shading delayed leaf senescence by increasing antioxidant enzyme activity and reducing lipid peroxidation, thereby supporting a relatively higher Pnin late-season leaves. Since the late season is an important period for seed filling and seed weight formation, the increased seed weight under light shading was possibly due to delayed leaf senescence and the resulting maintenance of a relatively high Pn. Moderate and severe shading also delayed leaf senescence, but their photosynthetic rates were much lower than those under light or non-shading due to insufficient light intensity, which resulted in reduce photosynthetic production and seed yield.
4.2. Increased seed yield was mainly due to an increase in hundred-seed weight under light shading
As expected, moderate and severe shading decreased the average seed yield by 9.8 and 33.3% in the present study as previously reported for cotton, soybean, and other crops (Savin and Slafer 1991; Zhao and Ooterhuis 2000; Proulx and Naeve 2009). However, light shading increased seed yield by 9.7%. Although the yield increment in oil-seed peony was relatively lower than in shade-loving plant species like Coptis chinensis Franch, Pinellia ternata(Thunb.) Breit, and Houttuynia cordata Thunb (Meng et al. 2007; Peng 2007; Zhao 2015), our finding is of great practical significance in enhancing agricultural production.Crop yield is determined by yield components (Zhang et al.2015). The changes in seed yield due to shading were undoubtedly related to variation in yield components. The number of follicles per unit ground area (follicle density), the number of seeds per follicle, and seed weight are the three major components of seed yield in oilseed peony. Our data indicated that moderate and severe shading reduced follicle density, and severe shading also reduced seed weight.Light shading increased the seed weight, but did not change the follicle density or the number of seeds per follicle. The results suggested that the increased seed yield under light shading was mainly due to an increase in seed weight, while the reduced yield under moderate and severe shading was attributed to low seed weight and follicle density.
4.3. Light shading improved seed nutritional quality through enhanced accumulation of crude fat and UFAs
Seed quality is determined by the proportion of starch,protein, and fat and its intrinsic quality, which is usually affected by environmental factors such as light intensity or temperature. A negative correlation was reported between protein content and light intensity during the early or middle period of grain-filling in wheat (Benzian and Lane 1986).Dong et al. (2014) also reported an increase in protein content and decreases in soluble sugar and crude fat contents in wheat grains under low light. Similar results were reported for maize under shading from joining to flowering stages (Zhang 2005). Therefore, it is generally believed that shading mostly improves the protein content and reduces fat content in starchy seeds. However, the shading effects on compositions of soybean seeds and other oil seeds seem to be more complex. In soybean, shading reduced the protein content and increased the crude fat content (Liu et al. 2009),or reduced yield and seed size with no effect on protein content (Lawn and Brun 1974). In other reports, it reduced yield without affecting the seed size, protein, or crude fat(Andrade and Ferreiro 1996), or with variable effects on seed size, protein, and crude fat (Wahua and Miller 1978).In our study, although shading had significant effects on seed composition, the effects varied with shading levels. The contents of crude fat and unsaturated fatty acid increased considerably under light shading, being consistent with an earlier report (Liu et al. 2016) of a quadratic relationship between light intensity and fatty acid content in soybean.
Seed composition is affected by “source-sink” relations(Proulx and Naeve 2009). The products of photosynthesis from leaves (the source) are transported to fruits, seeds,and roots (the sink) in the form of soluble sugars (Slewinski and Braun 2010). The soluble sugar is then transformed to starch, protein, and fat through a series of physiological and biochemical processes and stored in the sink (Desnoues et al. 2014; Peuke et al. 2015). A large source and small sink favoured the accumulation of crude fat (Wang et al. 1999).In the current study, the number of follicles and seeds per follicle was not significantly changed under light shading,but photosynthetic production as indicated by increased Pnwas improved, which might be an important reason for the increased content of crude fat. The composition of fatty acid is determined by differential expression of related genes like desaturase gene (Kodama et al. 2000; Li et al. 2015a), which is affected by a number of environmental factors (Heppard et al. 1996; Klyachko-gurvich et al. 1999; Guihéneuf et al.2015). In the present study, shading reduced the high light intensity and air temperature. The change in micro-climate would result in differential expression of fatty acid desaturase genes, thereby altering the content of unsaturated fatty acid. Further investigation of gene expression would be necessary to document the underlying mechanism of seed quality improvement under light shading.
5. Conclusion
Our study showed that the yield and quality of seed in oilseed peony were improved by light shading, while they were significantly impaired by moderate or severe shading. Light shading increased the activities of SOD and CAT, reduced the accumulation of H2O2and MDA, and thus delayed leaf senescence as indicated by the increased Pnand total Chl. Light shading increased seed weight, but moderate or severe shading reduced seed weight or follicle density.The increased yield under light shading was mainly due to an increase in seed weight, while the reduced yield under moderate or severe shading was mainly due to reduced follicle density and seed weight. Light shading also increased the contents of crude fat and unsaturated fatty acid. Thus, light shading is beneficial to seed yield and quality attributes, although it is generally believed that oilseed peony is not a shade-loving crop. Cultivating oilseed peony under an incomplete closed forest to provide a light shading environment might be a promising practice for increasing total output and income per unit land area.
Acknowledgements
This work was supported by the special fund for Taishan Scholars, Shandong Provincial Government, China(tspd20150213), Shandong Provincial Natural Science Foundation, China (ZR2017BC034) and the Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (Technology Integration and Demonstration of Oilseed Peony), China.
Ad?o R C, Glória M B A. 2005. Bioactive amines and carbohydrate changes during ripening of ‘Parta’ banana(Musa acuminata×M. balbisiana). Food Chemistry, 90,705–711.
Andrade F H, Ferreiro M A. 1996. Reproductive growth of maize,sunflower and soybean at different source levels during grain filling. Field Crops Research, 48, 155–165.
Bell G E, Danneberger T K. 1999. Temporal shade on creeping Bentgrass Turf. Crop Science, 39,1142–1146.
Bellaloui N, Bruns H A, Abbas H K, Mengistu A, Fisher D K,Reddy K N. 2015. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Frontiers in Plant Science, 6, 31.
Benzian B, Lane P W. 1986. Protein concentration of grain in relation to some weather and soil factors during 17 years of English winter-wheat experiments. Journal of Science of Food Agriculture, 37, 435–444.
Boydak E, Alpaslan M, Hayta M, Ger?ek S, Simsek M. 2002.Seed composition of soybeans grown in the Harran Region of Turkey as affected by row spacing and irrigation. Journal of Agricultural & Food Chemistry, 50, 4718–4720.
Chen Y Z, Dong H Z. 2016. Mechanisms and regulation of senescence and maturity performance in cotton. Field Crops Research, 189, 1–9.
Desnoues E, Gibon Y, Baldazzi V, Signoret V, Génard M,Quilot-turion B. 2014. Profiling sugar metabolism during fruit development in a peach progeny with different fructose-toglucose ratios. BMC Plant Biology, 14, 336.
Dong C, Fu Y M, Liu G H, Liu H. 2014. Low light intensity effects on the growth, photosynthetic characteristics, antioxidant capacity, yield and quality of wheat (Triticum aestivum L.)at different growth stages in BLSS. Advances in Space Research, 53, 1557–1566.
Dong H Z, Niu Y H, Kong X Q, Luo Z. 2009. Effects of earlyfruit removal on endogenous cytokinins and abscisic acid in relation to leaf senescence in cotton. Plant Growth Regulation, 59, 93–101.
Dong H Z, Niu Y H, Li W J, Zhang D M. 2008. Effects of cotton rootstock on endogenous cytokinins and abscisic acid in xylem sap and leaves in relation to leaf senescence. Journal of Experimental Botany, 59, 1295–1304.
Gindaba J, Wand S J E. 2005. Comparative effects of evaporative cooling, kaolin particle film, and shade net on sunburn and fruit quality in apples. HortScience, 40,592–596.
Guihéneuf F, Mimouni V, Trembling G, Ulmann L. 2015. Light intensity regulates LC-PUFA incorporation into lipids of Pavlova lutheri and the final desaturase and elongase activities involved in their biosynthesis. Journal of Agricultural and Food Chemistry, 63, 1261–1267.
Han C J, Meng Q H, Chen X M, Zhang C, Dong H Z, Zhang Y Z. 2015. Research and utilization progresses and industrial development strategies of oilseed peony in China.Shandong Agricultural Sciences, 47, 125–132. (in Chinese)
He X L. 2005. Studies on the photosynthetic characteristics of field tree peony. MSc thesis, Shandong Agricultural University, China. (in Chinese)
Heppard E P, Kinney A J, Stecca K L, Miao G H. 1996.Developmental and growth temperature regulation of two different microsomal ω-6 desaturase genes in soybeans.Plant Physiology, 110, 311–319.
Hong D Y, Pan K Y. 1999. Taxonomical history and revision of Paeonia section Moutan (Paeoniaceae). Acta Phytotaxonomica Sinica, 37, 351–368. (in Chinese)
Klyachko-Gurvich G, Tsoglin L N, Douche J, Kopetskii J,Shebalina I B, Semenenko V E. 1999. Desaturation of fatty acids as an adaptive response to shifts in light intensity.Physiologia Plantarum, 107, 240–249.
Kodama H, Nishiuchi T, Seo S, Ohashi Y, Iba K. 2000. Possible involvement of protein phosphorylation in the woundresponsive expression of Arabidopsis plastid omega-3 fatty acid desaturase gene. Plant Science, 155, 153–160.
Lawn R J, Brun W A. 1974. Symbiotic nitrogen fixation in soybeans. I. Effect of photosynthetic source-sink manipulations. Crop Science, 14, 11–16.
Li S S, Wang L S, Shu Q Y, Wu J, Chen L G, Shao S, Yin D D. 2015a. Fatty acid composition of developing tree peony(Paeonia section Moutan DC.) seeds and transcriptome analysis during seed development. BMC Genomics, 16,208.
Li S S, Yuan R Y, Chen L G, Wang L S, Hao X H, Wang L J, Zheng X C, Du H. 2015b. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section moutan DC.) cultivars by GCMS. Food Chemistry, 173, 133–140.
Li Y C. 2014. The strategy on the oil tree peony industry in China. Engineering Sciences, 16, 58–63. (in Chinese)
Liu B, Wang C, Jin J, Liu J D, Zhang Q Y. 2009. Effect of light enrichment and shading during reproductive stage on dry matter distribution, yield and quality of soybean. Agricultural Research in the Arid Areas, 27, 103–107. (in Chinese)
Liu J, Yang C Q, Zhang Q, Lou Y, Wu H J, Deng J C, Yang F, Yang F, Yang W Y. 2016. Partial improvements in the flavour quality of soybean seeds using intercropping systems with appropriate shading. Food Chemistry, 207,107–114.
Luzzi A F, James W P T. 2001. European diet and public health: The continuing challenge. Public Health Nutrition,4, 275–292.
Meng X H, Zhang Y J, Zhang H Q, Lu Y C, Yue J. 2007.Effects of shading treatment on biological characteristics of Pinellia ternata (Thumb.) Breit. Journal of Northwest A& F University, 35, 219–222. (in Chinese)
Ministry of Health of the People’s Republic of China. 2011.Announcement No. 9. (in Chinese)
Peng C H. 2007. Evaluation on the quality of different Houttuynia cordata and its regulated effect by shading treatment. MSc thesis, Fujian Agriculture and Forestry University, Fuzhou.(in Chinese).
Peuke A D, Gessler A, Trumbore S, Windt C W, Homan N,Gerkema E, As H V. 2015. Phloem flow and sugar transport in Ricinus communis L. is inhibited under anoxic conditions of shoot or roots. Plant, Cell and Environment, 38, 433–447.
Proulx R A, Naeve S L. 2009. Pod removal, shade, and defoliation effects on soybean yield, protein, and oil.Agronomy Journal, 101, 971–978.
Savin R, Slafer G A. 1991. Shading effects on the yield of an Argentinean wheat cultivars. Journal of Agricultural Science,116, 1–7.
Sevim D, Senol F S, Gulpinar A R, Orhan I E, Kaya E, Kartal M,Sener B. 2013. Discovery of potent in vitro neuroprotective effect of the seed extracts from seven Paeonia L. (peony)taxa and their fatty acid composition. Industrial Crops and Products, 49, 240–246.
Shi G A, Jiao F X, Jiao Y P, Yang H A, Han M W, Wu Y Q, Shi B R. 2014. Development prospects and strategies of oil tree peony industry in China. Journal of Chinese Cereals and Oils Association, 29, 124–127. (in Chinese)
Slewinski T L, Braun D M. 2010. Current perspectives on the regulation of whole-plant carbohydrate partitioning. Plant Science, 178, 341–349.
Wahua T A T, Miller D A. 1978. Effects of shading on the N2-fixation, yield, and plant composition of field-grown soybeans. Agronomy Journal, 70, 387–392.
Wang G H, Liu X B, Yang S P, Li Y H, Jin J, Zhang Q Y. 1999.Effects of source sink changes on grain yield and quality of soybean during reproductive growth period. Soybean Science, 16, 236–241. (in Chinese)
Xue J Q, Wang S L, Zhang P, Zhu F Y, Ren X X, Liu C J,Zhang X X. 2015. On the role of physiological substances,abscisic acid and its biosynthetic genes in seed maturation and dormancy of tree peony (Paeonia ostii ‘Feng Dan’).Scientia Horticulturae, 182, 92–101.
Zhang J W. 2005. Effects of light and temperature stress on physiological characteristics of yield and quality in maize.Ph D thesis, Shandong Agricultural University, China. (in Chinese)
Zhang Y, Liu A Z. 2016. The correlation between soluble carbohydrate metabolism and lipid accumulation in castor seeds. Biotechnology Bulletin, 32, 120–129. (in Chinese)
Zhang Y J, Chen Y Z, Lu H Q, Kong X Q, Dai J L, Li Z H, Dong H Z. 2016. Growth, lint yield and changes in physiological attributes of cotton under temporal waterlogging. Field Crops Research, 194, 83–93.
Zhang Y J, Song X Z, Yang G Z, Li Z H, Lu H Q, Kong X Q,Eneji A E, Dong H Z. 2015. Physiological and molecular adjustment of cotton to waterlogging at peak-flowering in relation to growth and yield. Field Crops Research, 179,164–172.
Zhao D, Ooterhuis D M. 2000. Cotton responses to shade at different growth stages: Growth, lint yield and fiber quality.Experimental Agriculture, 36, 27–39.
Zhao N. 2015. Study about effects of shading on growth and quality of Coptis Chinensis Franch. MSc thesis, Southwest University, China. (in Chinese)
Zheng G S, He X L. 2006. Studies on the photosynthetic improvement in the leaves of field tree peony through shading treatment in summer. Scientia Silvae Sinicae, 42,27–32. (in Chinese)
Zhou M, Zhao Y, Gai S P, Zheng G S. 2008. Comparison of chlorophyll fluorescence characters in tree peony leaves among three different senescence types. Shandong Agricultural Sciences, 3, 43–47. (in Chinese)
Zhou S G, Kong X S, Zhang M X, Wang L Y, Wang F Y, Zhou G Q. 2010. Effects of shading on photosynthesis, and other physiological and biochemical characteristics in tree peony.Scientia Silvae Sinicae, 46, 56–60. (in Chinese)
Zhu Y, Song H, Zhao S W, Wang L Y. 2012. Effect of shading treatment on photosynthetic characteristics and flower quality of Paeonia Suffruticosa. Acta Botanica Boreal-Occidentalia Sinica, 32, 0731–0738. (in Chinese)
Zhu Z H, Duan L S, Feng X M, Han B W, He Z P. 1998.Systematical analysis of hormones regulation on wheat leaves senescence. Acta Agronomica Sinica, 24,176–181.(in Chinese)
 Journal of Integrative Agriculture2018年7期
Journal of Integrative Agriculture2018年7期
- Journal of Integrative Agriculture的其它文章
- Characterisation of pH decline and meat color development of beef carcasses during the early postmortem period in a Chinese beef cattle abattoir
- Multi-mycotoxin exposure and risk assessments for Chinese consumption of nuts and dried fruits
- Effects of 1-methylcyclopropene and modified atmosphere packaging on fruit quality and superficial scald in Yali pears during storage
- Insertion site of FLAG on foot-and-mouth disease virus VP1 G-H loop affects immunogenicity of FLAG
- Update of Meat Standards Australia and the cuts based grading scheme for beef and sheepmeat
- Effects of leaf removal and cluster thinning on berry quality of Vitis vinifera cultivars in the region of Weibei Dryland in China
