Phenological stage effect on phenolic composition and repellent potential of Mentha pulegium against Tribolium castaneum and Lasioderma serricorne
Nidhal Salem, Jazia Sriti, Olfa Bachrouch, Kamel Msaada, Saber Khammassi, Majdi Hammami, Saoussen Selmi, Emna Boushih, Marwa Ouertani, Nesrine Hachani, Manef Abderraba, Brahim Marzouk, Ferid Limam,Jouda Mediouni Ben Jemaa
1Laboratoire des Substances Bioactives, Centre de Biotechnologie à la Technopole de Borj Cedria, BP, 901, 2050 Hammam Lif, Tunisia
2Laboratoire de Protection des Végétaux, Institut National de la Recherche Agronomique de Tunisie (INRAT), Rue Hedi Karray, 2049 Ariana, Tunis,Université de Carthage, Tunisia
3Institut Supérieur des Sciences Biologiques Appliquées de Tunisia
4Laboratoire de Biotechnologie Appliquée à l'Agriculture, Institut National de la Recherche Agronomique de Tunisie (INRAT), Rue Hedi Karray, 2049 Ariana, Tunis, Université de Carthage, Tunisia
1. Introduction
Despite the importance of the majority of insects for humanity as producers of pharmacologically active compounds such as venoms or antibodies, coleopterans constitute a real threat and a serious risk to the world. In fact, these insects are often found infesting food ingredients where they cause damage by piercing the packages, leading to grain deterioration and mycotoxin generation. The damaged products then become unfit for human consumption and must be removed from the food production chain[1,2].
The small reddish brown coleopteran [Tribolium castaneumHerbst(T. castaneum)] (Tenebrionidae) constitutes the major beetle of preserved grain throughout the world. The easy adaptability to survive harsh environments allows it to colonize different biotic and abiotic surfaces[3]. The tobacco pest,Lasioderma serricorne(L. serricorne) is a species of beetle insects of Anobiidae family. It is very similar to the worm of bread (Stegobium paniceum) and the little vrillette (Anobium punctatum). This insect causes damage in many herbs and spices[4].
Damage inflicted by these insects could be overcome by pesticides.However, synthetic insecticides may pose possible health hazards to human and environment[5]. Indeed, the repeated treatments of these chemical products may cause insecticide resistance in beetles[6]. In another hand, humanity is facing to severe threat from pesticides overusing. In fact, their drawbacks were coupled with neurotoxicity damages and neurodegenerative disorders by interfering with chemical neurotransmission, leading to lethal consequences[7,8].
As a result, an increasing attention was turned in developing better alternatives to synthetic insecticides. With respect to biological control,plant-based extracts have been suggested to provide potential insectcontrol agent. In this context, plant extracts contain complex mixture of secondary metabolites and natural dyes such as phenols which act as repellents, feeding deterrents and toxins[9].
Phenols such as quinine, lignin and salicylates are among the bestknown compounds tested against insects. The defensive mechanism of these compounds consists of limiting pathogens entrance by increasing the leaf toughness. Moreover, phenol oxidation activated by peroxidase would be considered as the ultimate defense against these pests[10].Of the same, flavonoids (such as flavonols, flavones, flavan 3-ols,flavonones, and isoflavonoids) and proanthocyanidins constitute other offensive agents against insects. Thanks to their cytotoxicity, enzymatic complexation and astringency, these phenolic compounds act as feeding deterrents and preventing attack from many phytophagous insects[10].
In Tunisia, several herbs can act as sources of these natural repellents,including pennyroyal [Mentha pulegiumL. (M. pulegium); Lamiaceae].This plant, known as Fliou, is one of the species frequently used in aromatherapy science among four other mint varieties:Mentha rotundifolia,Mentha longifolia,Mentha spicataandMentha aquatica[11]. Aerial parts ofM. pulegiumwere characterized by a wide diversity of bioactive components such as tannins, resins, pectins,polyphenols and essential oils. These constituents exhibited interesting biological activities such as antispasmodic, antihaemorrhoidal, sedative,hypotensive, anti-inflammatory, analgesic, antimicrobial and insecticidal activities[12].
Since there are no published reports concerning the effect of phenological stage on the insecticidal potentials ofM. pulegiumextracts until now, this study was undertaken. Hence, the common target of this research was to ascertain the potential impact of phenological stages on polyphenol, flavonoid and proanthocyandin contents and their antioxidant activity (i), to determine the profiles of different phenolic compounds and quantify them (ii) and to assess the repellent potentials ofM. pulegiumextracts againstT. castaneumandL. serricorneduring its vegetable stages (iii). Such a work is encouraging in order to recommend the use ofM. pulegiumas a safe, convenient,and low-cost alternative bioinsecticide instead of harmful pesticides.
2. Materials and methods
2.1. Chemical reagents
The reagents used were sodium hydroxide (NaOH), chlorhydric acid(HCl), disodium hydrogen phosphate (Na2HPO4), sodium monobasic phosphate (NaH2PO4H2O), sodium carbonate (Na2CO3), sodium nitrite(NaNO2), butylated hydroxytoluene (BHT), 1,1-diphenyl-2-picrylhydrazyl(DPPH), potassium ferricyanide [K3Fe(CN)6], trichloroacetic acid (TCA),ferric chloride solution, ascorbic acid, polyvinyl polypyrolidone Folin Ciocalteu reagent, potassium ferricyanide [K3Fe(CN)6] and aluminum chloride (AlCl3). All these products were provided from Sigma Aldrich(Steinheim, Germany). Phenolic authentic standards were purchased from Fluka. Stock solutions of these compounds were prepared in high performance liquid chromatography (HPLC)-grade methanol, conserved in aluminum glass vials and stored at -20 ℃. Methanol used was of analytical grade and delivered from laboratory reagents and fine chemicals.
2.2. Vegetal collection
To ensure homogeneous sampling,M. pulegium(leaves, stems and flowers) were collected randomly from Oued el Abid region [latitude 36o 52’ 52.19’’(N); longitude 10o 42’ 26.41’’(E), altitude 1060 m)] at three phenological stages; vegetative (ST1), full flowering(ST2) and fructification (ST3) stages. The collection was done three times. The plants were identified by the botanist Abderrazak (Professor in Biotechnology Center in Borj Cedria Technopole, Tunisia)according to the Tunisian flora[13]. We prepared the voucher specimen in laboratory of bioactive substances for further studies. In presence of nitrogen, pennyroyal samples were freeze-dried and ground to fine powder. After that, they were conserved in a dessicator at room temperature (~25 ℃) in darkness for later use.
2.3. Phenol extraction
The dried samples were milled in an electrical stainless steel blender and placed at room temperature away from moisture. Concerning the extraction by Soxhlet, a quantity of 20 g of vegetable powder (aerial part) were placed in a cartridge in the presence of 200 mL of methanol.The extraction lasted 8 h. The extract was recovered, filtered (Wattman# 4), concentrated by rotary evaporator and stored at 4 ℃.
2.4. Total polyphenol content
According to the extraction protocol described by Dewantoet al[14].Quantitative estimation of polyphenols was carried out using the Folin ciocalteu reagent. Different concentrations of stock solution were prepared. For each one was added to 125 μL of Folin Ciocalteu reagent. After 2 min, 1250 μL of Na2CO3(7 g/100 mL) were supplied to promote an alkaline medium and to start the oxidation-reduction reaction. After adjusting with distilled water to a final volume of 3 mL, these extract solutions were kept in the dark for 90 min at room temperature. The absorbance of each extract solution was read as described at 760 nm.
Gallic acid was used as a standard to estimate the total amount of polyphenols.
2.5. Total flavonoid content
The flavonoid assay was performed by a method based on the formation of complex between phenolic compounds and AlCl3[14].From the stock solution of pennyroyal extracts prepared in methanol,different dilutions ranging from 25 to 500 μg/mL were prepared. A total of 0.125 mL of each solution was added to 75 μL of NaNO2solution (5%) and 0.15 mL of AlCl3(10%). All were mixed thoroughly for 6 min. Obtained mixture was then added to 0.5 mL of 1 M NaOH solution. The assay was carried out by UV/Visible spectrophotometry at 510 nm and total flavonoid content of pennyroyal aerial parts was expressed as mg quercetin equivalents (QE) per gram of dry weight (mg QE/g DW) through its calibration curve.
2.6. Proanthocyanidin content
The colorimetric method was based on the oxidative cleavage of proanthocyanidins with hydrochloric acid showing a red color proportional to the quantity of these compounds[15]. A volume of 0.5 mL of methanolic extract was added to a volume of 3 mL of vanillin solution (4%) and 1.5 mL of HCl. After 15 min, the absorbance was read at 500 nm and the concentration of proanthocyanidins was expressed in quercetin equivalent (mg QE/g DW).
2.7. Phenolic profile analysis of pennyroyal extracts by RPHPLC
Twenty μL of pennyroyal extracts were analyzed on a HPLC system (Agilent 1260, Agilent technologies, Germany). This method consisted on a vacuum degasser, an autosampler and a binary pump with a maximum pressure of 400 bar. The phenolic compounds were separated on a reversed phase C18 analytical column (Zorbax Eclipse XDB, 4.6 mm × 100 mm, 3.5 μm particle size) at a temperature set at 25 ℃. The chromatograms obtained at 280, 320 and 550 nm (DAD detector, Germany) were compared with those obtained on known phenolic compound standards. The separation gradient was formed by two mobile phases: A, methanol and B, 99.9% H2O and 0.1% formic acid. The optimized gradient elution was illustrated as follows: 0-5 min,10%-20% A; 5-10 min, 20%-30% A; 10-15 min, 30%-50% A; 15-20 min, 50%-70% A; 20-25 min, 70%-90% A; 25-30 min, 90%-50% A;30-35 min. Phenolic compound contents were expressed in mg/g DW.
2.8. Antioxidant capacity evaluation
2.8.1. Antiradical scavenging potential
In this analysis, the purple-colored DPPH was reduced by the socalled antioxidants into pale yellow hydrazine. So antioxidant effect on this radical translated its ability to give hydrogen radical[16]. Two thousand μL of each methanolic extract at different concentrations(0.1-100 μg/mL) were added to 500 μL of a methanolic solution from DPPH at 0.2 mM. After incubation in the dark for 30 min and at room temperature, the reading of absorbances was carried out at 517 nm using a spectrophotometer.
We thus calculated the percentages of inhibition by the following formula:
IP% = [(Ac-At)/Ac]×100
Where IP% was inhibition percentage, Ac absorbance of control and At absorbance of the test made. BHT was used as positive control at the same concentrations as the tested extracts.
The semi-logarithmic curve obtained made it possible to establish the IC50of the sample which corresponded to the concentration leading to 50% of DPPH radical inhibition.
2.8.2. Ferric reducing antioxidant power
The reducing power of an extract was associated with its antioxidant power. The reducing ability of our extracts was determined according to the method of Oyaizu (1986)[17]. This method was based on the chemical reaction to reduce ferric iron (Fe3+) present in the complex K3Fe(CN)6into ferrous iron (Fe2+). To 1 mL of the sample at different concentrations (50, 200, 1000 and 2000 μg/mL), 2.5 mL of a phosphate buffer solution (0.2 M, pH 6.6) and 2.5 mL of K3Fe(CN)6at 1% were added. The mixture was incubated at 50 ℃ for 20 min and then cooled at room temperature. About 2.5 mL of TCA (10%) was added to stop the reaction, then the tubes were centrifuged at 3000 rpm for 10 min. Then 2.5 mL of the supernatant were added to 2.5 mL of distilled water and 500 μL of a 0.1% solution of FeCl3?6H2O.The absorbances were read against a blank at 700 nm using a spectrophotometer. An increase in the absorbance corresponded to an increase in the reducing power. So, the concentration providing 0.5 of absorbance (EC50, μg/mL) was calculated directly from the graph of absorbance. Ascorbic acid was used as a positive control in this experiment under the same conditions.
2.9. Insecticidal activity
2.9.1. Pest rearing
The experiment was carried out in the laboratory of plant protection at the National Institute of Agricultural Research in Tunisia on two strains;the red floor beetleT. castaneumand the cigarette beetleL. serricorne. This choice of animal material was justified by the importance of the damage of these pests, which infested stored foodstuffs of economic importance.These insects were easily reared in the laboratory in glass jars on wheat floor (Triticum aestivum) without exposure to insecticides. All these jars were placed in a dark oven set at a temperature of (25±1) ℃, a relative humidity of (65±5)% and 15 h:9 h (light: dark) photoperiod, which were the optimal conditions for the development of these insects. It should be noted that pests used for repellent testing were 7-10 days old.
2.9.2. Repellency bioassay
A single insecticidal test was assessed for the extracts during different stages ofM. pulegiumagainst two beetle species:T. castaneumandL.serricorne
This test was used to study the repulsive effect of the extract tested against the two beetles by determining the percentage of repulsion of each extract as well as the median dose of repulsion. The repellent effect ofM. pulegiumextracts on coleopteran was evaluated using the preferential filter paper method described by Jilani and Saxena[18].Thus, the 8 cm diameter Wattman filter paper discs were used. For this purpose, they were cut into two equal parts. Four concentrations of extracts were prepared from the stock solution (Ss) [C1 (Ss), C2 (1/2 Ss), C3 (1/4 Ss), and C4 (1/8 Ss)] by dilution in acetone. Then 0.150 mL of each dilution thus prepared was spread uniformly over one half of the disc while the other half received only 0.150 mL of acetone.Five repetitions were performed for each concentration. After fifteen min which was time required for complete evaporation of solvent,the two halves of the disks were attached using an adhesive tape. The reconstituted filter paper disc was placed in a petri dish and 20 insects of mixed sex (ratio 1:1) were placed in the center of each disc.
The mortality monitoring of the insects on the petri dish was carried out according to a kinetics of 1 h up to 24 h for each tested concentration.
Number of insects (L. serricorneandT. castaneum) presented on the part of filter paper treated with pennyroyal extract (Nt) and the number of those presented on the part treated only with acetone (Nc) were recorded. The percentage of repulsion (PR) was calculated using the formula of Nerioet al[19] as bellow:
PR = [(Nc-Nt)/(Nc+Nt)] × 100
The mean repellency value of each extract was calculated and assigned to repellency classes from 0 to Ⅴ.
Class 0: The percentage of repulsion ≤ 0.1%: not repulsive
Class Ⅰ: 0.1%<PR≤20%: very poorly repulsive
Class Ⅱ: 20.1%<PR≤40%: weakly repulsive
Class Ⅲ: 40.1%<PR≤60%: moderately repulsive
Class Ⅳ: 60.1%<PR≤80%: repulsive
Class Ⅴ: 80.1%<PR≤100%: very repulsive[20].
Probit analysis[21] was used to calculate the median repellent dose(RD50) (dose that repelled 50% of the exposed insects) and RD95(dose that repelled 95% of the exposed insects) at 24 h of exposure. Results were presented as the mean of percentage repellency ± the standard error.
2.10. Statistical analysis
The results obtained were analyzed statistically using STATISTICA software[22] and expressed as mean ± SD. Then, they were discriminated against Duncan’s test at threshold of 5%. Probit analysis was conducted to estimate repellent doses (RD50and RD95)with their 95% fiducial limits. Correlation coefficients were calculated between total polyphenol, total flavonoids, proanthocyanidins and their antioxidant activities (DPPH and reducing power) based on their contents.
3. Results
3.1. Extraction yield
Extraction yield varied significantly (P<0.05) as a function of the development stage ofM. pulegium. In fact, higher extraction yield was reached at ST2 stage (19.34%), followed by that at ST1 stage (14.23%).Whereas, ST3 stage showed the lowest extraction yield with a value of 11.11%.
3.2. Phenological effect on phenolic contents
Phenolic quantification (polyphenols, flavonoids and proanthocyanidins)depending on harvest time was given by Table 1. Indeed, polyphenol level reached its maximum during the full flowering stage (ST2) with a content of 37.67 mg GAE/g DW. Beyond this stage, the level decreased to a value of 22.19 mg GAE/g DW.
Relative contribution of stage development on flavonoids and proanthocyanidins showed approximately the same classification as polyphenols. In fact, the maximum of flavonoid and proanthocyanidin levels were detected during the full flowering stage with respectively 164.28 mg QE/g DW and 4.80 mg QE/g DW (Table 1).
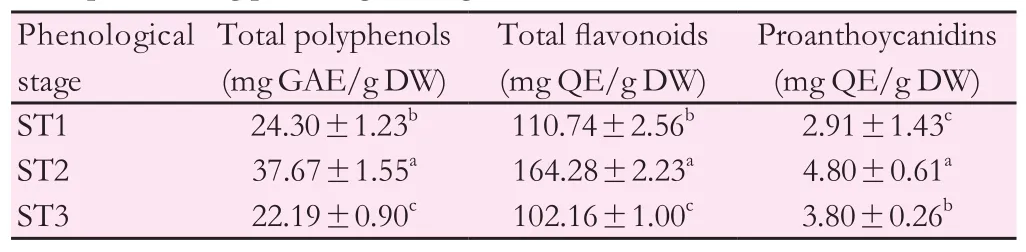
Table 1 Total polyphenol, flavonoid and proanthoyanidin contents of M. pulegium aerial parts during phenological stages.
3.3. Phenolic coumpounds profile
In order to elucidate metabolic variations of phenols at ST2 stage ofM.pulegium, the profile of individual phenolic compounds was investigated and presented in Table 2. Total phenols identified were (12.94 ± 0.18)mg/g DW. Apigenin was the main flavone, with value reaching up to 6.01 mg/g DW of the total phenols. Diosmin (1.92 mg/g DW),p-coumaric acid (1.60 mg/g DW) and rosmarinic acid (1.66 mg/g DW) were also considered as major phenolics of pennyroyal extracts.
Identified phenolic compounds were classified into five chemical classes with values varying from 0.31 to 8.12 mg/g DW.M. pulegiumextracts were rich in flavones [(8.12 ± 0.06) mg/g DW] and hydroxycinnamic acids [(3.26 ± 0.05) mg/g DW] which constituted the major classes during ST2 stage (Table 2). However, the percentages of other classes did not exceed [(0.57 ± 0.02) mg/g DW for flavanols, (0.68 ± 0.04) mg/g DW for flavonols and (0.31 ± 0.01) mg/g DW for flavanones].
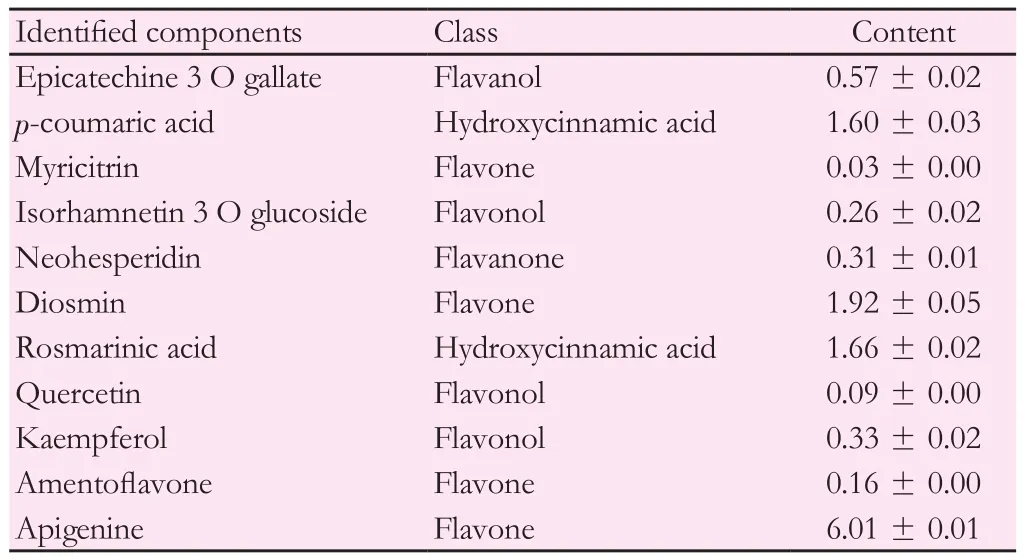
Table 2 Contents of individual phenolic compounds (mg/g DW) of M. pulegium aerial parts during flowering stage (by elution order).
3.4. Phenological effect on antioxidant capacity
In vitroantioxidant activity of pennyroyal extracts was investigated during its different vegetable stages using two different and complementary methods (DPPH and reducing power tests). Interestingly, allM. pulegiumextracts showed a scavenging ability on DPPH higher than that of commercial standard, BHT (IC50= 7.5 μg/mL) (Table 3).
Concerning the effect of phenological stage, changes in the antioxidant activities of various plant extracts were of great importance from the nutritional and pharmaceutical points of view. In this regard, radical scavenging activity of pennyroyal methanolic extracts showed an increasing ability to scavenge the DPPH radicals from ST1 (IC50=0.80 μg/mL) to ST3 (IC50= 0.09 μg/mL). Of the same, despite the moderate antioxidant activity by comparison to ascorbic acid [(42.00 ±0.05) μg/mL], lowest value of EC50was recorded at ST3 reflecting the maximum of reducing power activity during this period (EC50= 700 μ g/mL) (Table 3).
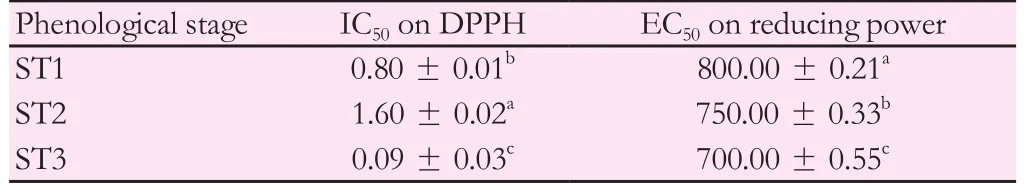
Table 3 Antioxidant properties against DPPH radical (IC50) and reducing power (EC50)of M. pulegium aerial parts (μg/mL).
3.5. Relationship between total phenols and antioxidant activity
Correlation coefficients for DPPH, reducing power, total polyphenol,flavonoid and proanthocyanidin contents were given in Figure 1 and Figure 2. According to these results, the antioxidant activity (in terms of free-radical scavenging activity and reducing power) did not show correlation with phenol contents.
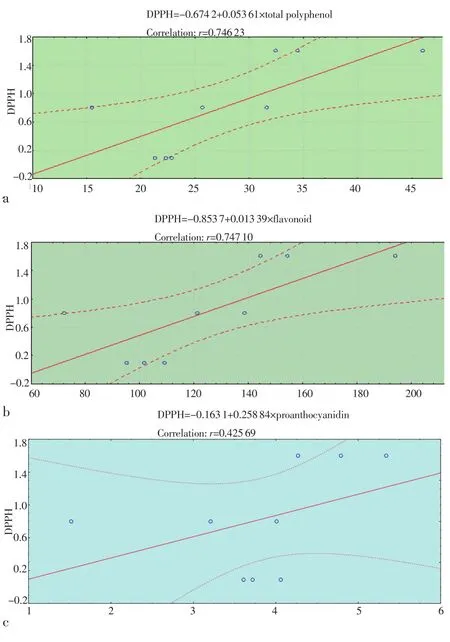
Figure 1. Correlations between free radical scavenging activity (test DPPH)and total polyphenol (a), flavonoid (b) and proanthocyanidin (c)contents.
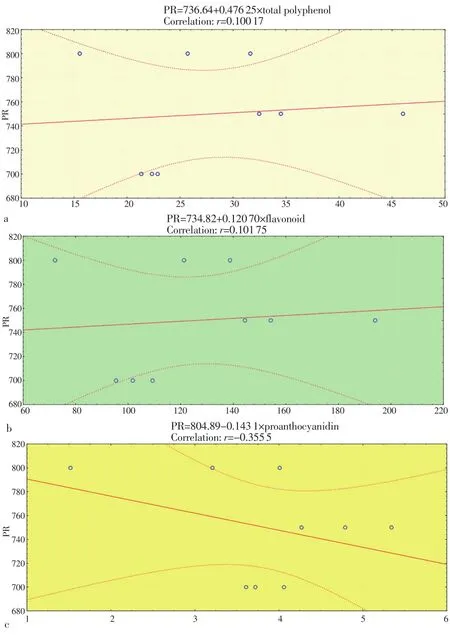
Figure 2. Correlations between reducing power activity and total polyphenol(a), flavonoid (b) and proanthocyanidin (c) contents.
3.6. Phenological effect on insecticidal activity
As demonstrated in Tables 4 and 5, the repellent potentials of pennyroyal methanolic extracts during phenological stages were evaluated. For this, three dilutions (1/2, 1/4 and 1/8) from the solution stock of 1 mg/mL were tested againstT. castaneumandL. serricorne.Pennyroyal extracts showed strong repellent efficacy against the two coleopteran pests used. At a given exposure time, the repellent effect was significant and proportional to the concentration of the extract. Indeed, the repelling effect of the lowest dilution (0.125 mg/mL) during ST1 stage againstT. castaneumwas 6.66% after 1 h of exposure and 53.33% after 24 h of exposure. Similarly, for the highest concentration (1 mg/mL), the percentage of repulsion increased from 20% after 1 h of exposure to 80% after 24 h of exposure (Table 4).Meanwhile, at the same doses, pennyroyal extracts reached only 20%and 56.66% repellency after 24 h of exposure againstL. serricornefor 0.125 and 1 mg/mL respectively (Table 5).
The insecticidal effect ofM. pulegiumextracts varied depending on the phenological stage for the two beetles. Indeed, for the highest concentration (1 mg/mL), the repellent effect againstT. castaneumreached its maximum (90%) during ST3 stage (after 24 h of exposure).However, this repellent effect was 80% during ST1 stage (Table 4).As forL. serricorne, the same trend was underlined where insecticidal potential was less pronounced at ST2 and ST3 stages compared to ST1 stage. In fact, during ST2 and ST3 stages, the repulsion percentages increased from 10% to 30% and 43.33%, respectively, for the highest concentration (1 mg/mL) after 24 h of exposure (Table 5).
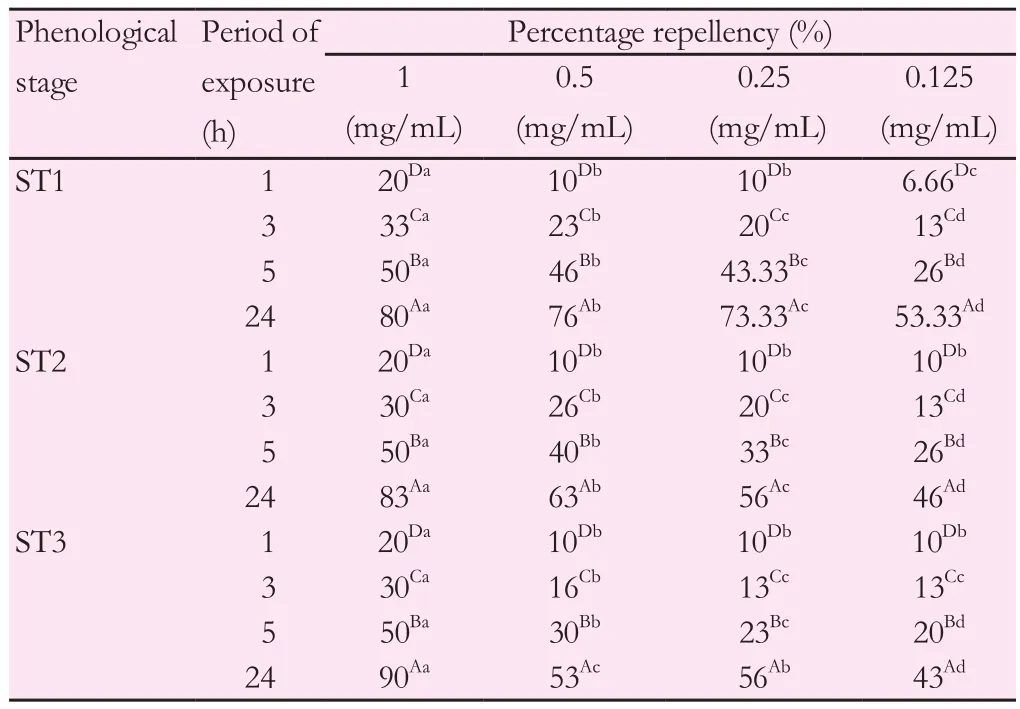
Table 4 Percentage repellency (%) of M. pulegium methanolic extracts against T.castaneum adults during phenological stages.
Comparison made between mean values from the same column (by letters in uppercase) revealed exposure time impact on insect repellency. Comparison made between mean values from the same row (by letters in lowercase)indicated oil concentration impact on insect repellency. Values followed by the same letter were not significantly different according to LSD test atP<0.05.(ST1): Vegetative stage, (ST2): Full flowering stage, (ST3): Fructification stage.
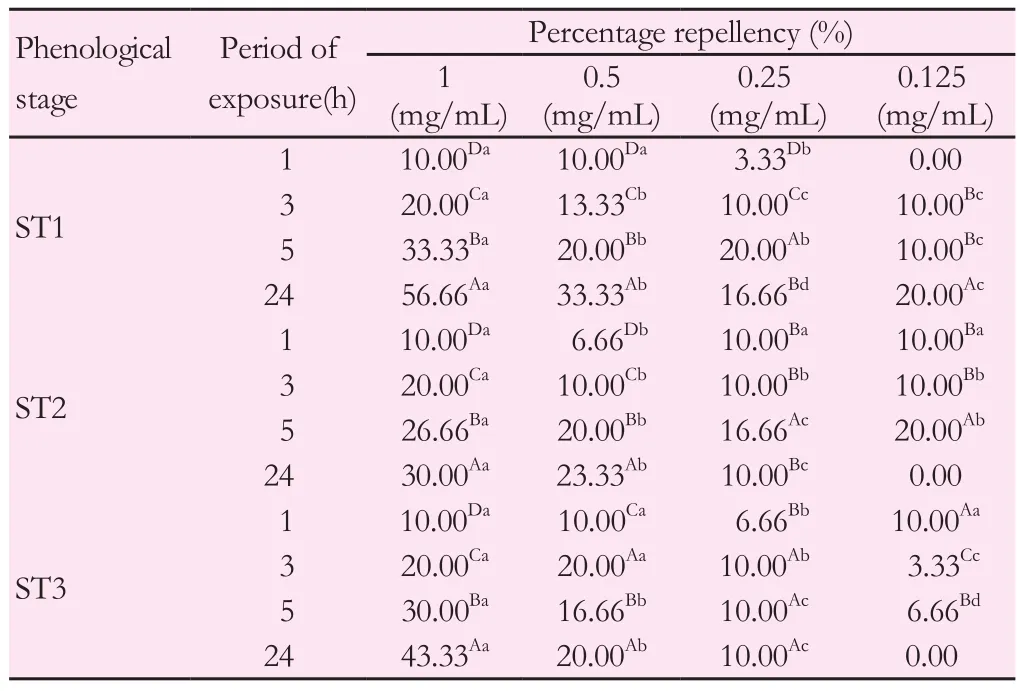
Table 5 Percentage repellency (%) of M. pulegium methanolic extracts against L.serricorne adults during phenological stages.
As mentioned in Tables 4 and 5, we classified the level of repellency ofM. pulegiumextracts into six categories. During ST1 stage, pennyroyal extracts showed class Ⅳ repellency status for 1 mg/mL, 0.5 mg/mL and 0.25 mg/mL doses (againstT. castaneum) after 24 h of exposure.However, this extract showed classes Ⅲ, Ⅱ and Ⅰ respectively during ST1 stage (againstL. serricorne) for the same doses.
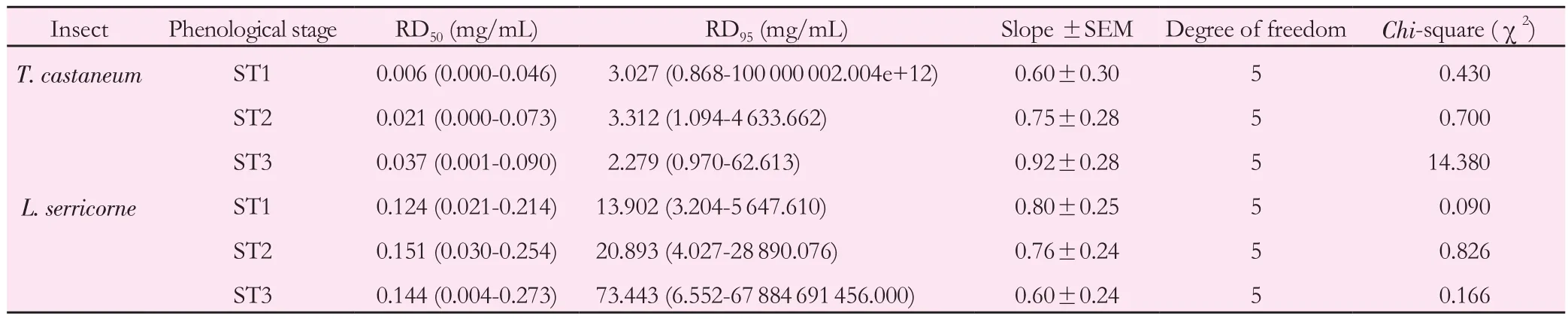
Table 6 Repellency doses RD50 and RD95 values of M. pulegium extracts against T. castaneum and L. serricorne adults after 24 h of exposure.
Probit analysis showed that results of Table 6 were also in line with those of Tables 4 and 5. The insecticidal activity of the two beetles was more pronounced during ST1 compared to ST2 and ST3 stages with the lowest repulsive doses ranging from 0.006 to 0.124 mg/mL for RD50and from 3.027 to 13.902 mg/mL for RD95respectively againstT. castaneumandL. serricorne. Furthermore, RD50and RD95values ofM. pulegiumextracts tested withL. serricornewere higher than those ofT. castaneum. Hence,we can note thatL. serricornewas more susceptible to pennyroyal extract thanT. castaneumadults.
4. Discussion
Interestingly, the Soxhlet extraction of pennyroyal aerial parts showed yield values better than those reported in the literature. Indeed, the study of Khadraouiet al[23] showed yields of 1.54%, 1.62% and 4.77%(w/w, calculated on dry matter) ofM. pulegiumleaves at ST2 stage.This extraction wad made by three different solvents which were respectively;n-butanol, ethyl acetate and petroleum ether. Thus, this difference could be explained by the fact that petroleum ether, ethyl acetate andn-butanol are non-polar solvents and they are used to degrease the drugs and to extract lipophilic compounds (lipids, fatty acids, chlorophylls,etc.). In addition, several other studies have shown that methanol could be the best solvent capable of providing the higher extraction yield[24,25] which explains our choice in the present work.
However, our results showed lower values than those found by Sarikurkcuet al[26]. Indeed, at the flowering stage, the yield of the methanolic extract ofM. pulegium, collected from Turkey, reached up 22.85%. This difference may be due to the seasonal and regional variations characterizing the two collecting regions of pennyroyal. The impact of other factors on the extraction yield could be reported in many other studies such as the type of solvent used and its coefficient of diffusion, the particle size and the extraction method[27].
Concerning phenolic contents, our findings were in agreement with those reported by Karray-Bouraouiet al[28] where phenolic composition ofM. pulegiumshoots from Tunisian (Bouarada) showed a polyphenol amount of 25 mg GAE/g DW at full flowering stage. On the other hand, a recent study lead by Sarikurkcuet al[26] focused on the composition of polyphenol contents and the antioxidant activity of pennyroyal aerial parts from Turkey which demonstrated lower polyphenol content (97.20 μg GAE/g DW). So, this variation may be due to the collection region effects[29]. Obtained results highlighted the richness of pennyroyal aerial parts in total flavonoids compared to other researches such as Sarikurkcuet al[26] and Khennoufet al[30]who determined levels of 20.88 μg QE/g DW and 35.16 μg QE/g DW respectively ofM. pulegiumgrown in Turkey and Algeria. These values were significantly lower than those found in this study, suggesting geographic variations related to climatic and/or soil factors[29]. As for proanthocyanidins, many other factors may affect these secondary metabolite productions such as intraspecific variations[31]. Thereby,there is no general trend for phenolics distribution depending on genotype and harvest time. However, the majority of mint species showed that the biosynthesis of the highest content of phenolic compounds was enhanced in the stage of full flowering, mainly during the time of ultraviolet radiation[32,33].
In agreement to our observations, several researchers have reported an increase in phenolic concentrations among different Lamiaceae species such as sweet marjoram (Origanum majoranaL.)[34],Lavandin(Lavandula×intermedia)[35] andMelissa officinalis[36]. Hence,according to Herms and Mattson[37], the concentrations of some secondary metabolites (particularly low molecular-weight phenolics) are synthesized only during early stages of seedling growth.
In terms of compositional analysis, HPLC analysis of pennyroyal extracts identified 11 phenolics owned to five chemical classes. These findings were in line with the recent study of Aireset al[38] where Apigenin was found as the major phenolic compound inM. pulegiumfrom Portugal. While differing for other mint species, rosmarinic and chlorogenic acids were found as the major identified compounds respectively inMentha spicataandMentha rotundifolialeaves collected from Algeria. These differences could be related to genetic,intraspecific factors and agroclimatic conditions[38]. For instance,apigenin is considered as a potential health promoting and functional food agent thanks to its numerous biological activities such as cytostatic and cytotoxic effects[39]. Of the same, this flavone is known as antioxidant, anti-inflammatory, anti-mutagenic, and anti-tumorigenic agent[40]. Therefore, the presence of high levels of this compound in pennyroyal makes this plant very promising as natural source of bioactive principles.
This may explain the high antioxidant and anti-inflammatory potentials during this period[41]. So, full flowering stage was determined as an ideal time to harvest the utmost contents of phenols from Tunisian pennyroyal.
The antioxidant activity of pennyroyal extracts was evaluated using DPPH test and reducing power ability. Results of our study showed that the antiradical activity of methanolic extracts was better than many otherMenthaspecies reported in the litterature such as;Mentha spicata(IC50= 87.89 μg/mL),Mentha rotundifolia(IC50= 21.71 μg/mL),Mentha longifolia(IC50= 24.07 μg/mL) andMentha piperita(IC50=13.32 μg/mL)[42]. In another hand, these extracts showed moderate ability to reduce the iron ions by comparison to ascorbic acid (EC50= 42 μg/mL). In this context, the researches of Sarikurkcuet al[26]and Khaled-Khodjaet al[43] have reported a reducing power ofM.pulegiumlower than the present work with respectively EC50of 1.224 mg/mL (from Turkey) and (427.5 ± 15.41) mg/mL (from Algeria).This variation may be explained by the extraction solvent effect,plant organ (leaves, fruit, bark,etc.) and geographical factors as well as the method used to determine the antioxidant activity. Moreover,this strong activity ofM. pulegiumcould be mainly attributed to its richness in flavonoids (as described above). In fact, it is believed that these compounds may act as potent free radical scavengers and reducing agents[44].
It should be noted that to the best of our knowledge, no report was undertaken on the antioxidant activity of pennyroyal aerial parts during phenological stages. However, investigations about other plants were reported. Indeed, as agreed with our results, Gru?ováet al[45] showed that the highest antioxidant activity of peppermint (Mentha piperita,Lamiaceae) leaves collected in Slovakia was determined at the end of growing season periodi.e.August–September (during fructification)(with IC50between 56.29–57.35 μg/mL). This increase may be due to climatic factors, mainly the low precipitation (12.4-24.8 mm) and the high temperatures. Meanwhile, a study conducted by Wanget al[46],showed an inverse trend compared with our findings. The antioxidant capacity (measured by FRAP assay) of jujube (Ziziphus jujubaMiller)fruits decreased during three edible maturity stages.
Statistical analysis with an objective to find any correlation between phenolic ontents and their antioxidant potential showed that the antioxidant activity does not only depend on the content of phenolic compounds but also on their nature (number of available hydroxyl groups and complexity of reaction kinetics) and the interactions between them[47].
In fact, according to several researchers, total extracts are complex mixtures of many different compounds which may be without a phenolic structure and distinct activities[42].
In contrast to our results, other reports showed that phenolic content could be used as an indicator of antioxidant properties of many plants.In fact, a positive correlation between polyphenols extracted fromSidastrum micranthumandWissadula periplocifoliacrude extracts and their antioxidant abilities was shown by De Oliveiraet al[48]. In addition, a high correlation was found between the DPPH scavenging potency and total phenolic contents of traditional plant species such asAllium sativumandPistacia lentiscus[49].
Several studies have demonstrated the risk of acute and chronic effects for farmers and consumers exposed to pesticides and their harmful side effects including respiratory, cutaneous, neurological and reproductive problems. For this reason, researchers are looking for other natural alternatives of plant origin. In this study, insecticidal effect ofM. pulegiumwas investigated and showed interesting dose and timedependent repellency effect against the two beetles;T. castaneumandL. serricorne.
As compared toMenthaessential oil, there is not any publication focusing on the repellent potential ofM. pulegiumaerial parts extracts against coleopteran insects. Nevertheless, insecticidal activities of other mint species extracts against different pests were reported by many previous studies. In fact, Mangalatet al[50] have reported the efficacy ofMentha rotundofolialeaves extracts against the mosquitoCulex pipiens(30% mortality) after 48 h of exposure. On a related field study, Pascual-Villalobos and Robledo[51] obtained a 0.05% repellency effect ofMentha longifoliahexane extract (after 2 h of exposure)againstT. castaneum. While, Regnault-Roger and Hamraoui[52] used dry material ofMentha piperitaand obtained a mortality of 47.7%againstAcanthoscelides obtectu. Hence, as compared to these species,M. pulegiumcould be considered as the most interesting natural insecticide. Likewise, its effectiveness could be related to its chemical composition.
In another hand, the repellency potential of plant extracts depends upon the extraction solvent. In fact, Pascual-Villalobos and Robledo[51]have found that solvent polarity has a positive impact on the extraction of bioactive components responsible for the insecticidal effect.Similarly, extraction process, the age of the targeted pest organism and the developmental stage of the plant used were among the factors that may influence the insecticidal effect[9,53,54].
In the light of these results, this study showed a good potential of the use ofM. pulegiumas natural repellent agent againstTriboliumandLasiodermainstead to synthetic pesticides. However, all these applications need further analyses with the intention of understanding the access mode of bioactive molecules responsible of the repellent activity of pennyroyal extract.
In conclusion, the determination of optimum conditions for phenol accumulation as well as their biological activities is an important field in order to valorizeM. pulegiumas source of natural phytochemicals.As a whole, our findings showed that polyphenol, flavonoid and proanthocyanidin contents and their antioxidant capacities depend on the plant stage of this medicinal plant. The fructification stage was considered as a proper harvesting period forM. pulegiumto have high antioxidant potentials. Of the same, the results highlighted the strong repellent capacity ofM. pulegiumextracts especially during early vegetable stage. Therefore, this source of natural products could be considered as a promising tool to replace synthetic insecticides.However an intensive research on the mechanism of insect action,a study of synergistic effects between different constituents of pennyroyal extract and the search of new additives are the important items to improve commercial and food repellent formulations.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
This topic was undertaken by a Project of Valorization of the Results of Research (VRR) and funded by the Ministry of Higher Education and Scientific Research in Tunisia.
[1] Deepak K, Prasanta K. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries.Foods2017; 6(1):8.
[2] Karlovsky P, Suman M, Berthiller F, De Meester J, Eisenbrand G, Perrin IP,et al. Impact of food processing and detoxification treatments on mycotoxin contamination.Mycotoxin Res2016; 32: 179-205.
[3] Nigussie M, Tanner D, Twumasi-Afriyie S.Enhancing the contribution of maize to food security in Ethiopia. Ethiopia: Central Printing Press PLC;2001.
[4] Phoonan W, Deowanish S, Chavasiri W. Food attractant from mulberry leaf tea and its main volatile compounds for the biocontrol ofLasioderma serricorneF. (Coleoptera: Anobiidae).J Stored Prod Res2014; 59: 299-305.
[5] Carvalho FP. Pesticides, environment, and food safety.Food Energy Secur2017; 6(2): 48-60.
[6] Scott IM, Tolman JH, Mac Arthur DC. Insecticide resistance and crossresistance development in Colorado potato beetleLeptinotarsa decemlineataSay (Coleoptera: Chrysomelidae) populations in Canada 2008-2011.Pest Manag Sci2015; 71(5): 712-721.
[7] Bisseleua HB, Gbewonyo SW, Obeng-Ofori D. Toxicity, growth regulatory and repellent activities of medicinal plant extracts onMusca domesticaL.(Diptera: Muscidea).Afr J Biotechnol2008; 7(24): 4635-4642.
[8] Lee I, Eriksson P, Fredriksson A, Buratovic S, Viberg H. Developmental neurotoxic effects of two pesticides: Behavior and neuroprotein studies on endosulfan and cypermethrin.Toxicology2015; 335: 1-10.
[9] Abtew A, Subramanian S, Cheseto X, Kreiter S, Garzia TG, Martin T.Repellency of plant extracts against the legume flower thripsMegalurothrips sjostedti(Thysanoptera: Thripidae).Insects2015; 6: 608-625.
[10] War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S,et al. Mechanisms of plant defense against insect herbivores.Plant Signal Behav2012; 7(10): 1306-1320.
[11] Pottier-Alapetite G.Flora of Tunisia angiosperms-dicotyledones gamopetales. Tunis, Tunisia: Official Printing Office of the Tunisian Republic; 1981, p. 812-814.
[12] Brahmi F, Hauchard D, Guendouze N, Madani K, Kiendrebeogo M,Kamagaju L, et al. Phenolic composition,in vitroantioxidant effects and tyrosinase inhibitory activity of three AlgerianMenthaspecies:M. spicata(L.),M. pulegium(L.) andM. rotundifolia(L.) Huds (Lamiaceae).Ind Crops Prod2015; 74: 722-730.
[13] Pottier-Alapetite G.Flora of Tunisia angiosperms-dicotyledones gamopetales. Tunis, Tunisia: Official Printing Office of the Tunisian Republic; 1979, p. 1074.
[14] Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity.J Agric Food Chem2002; 50: 3010-3014.
[15] Sun BS, Ricardo-Da-Silva JM, Spranger MI. Critical factors of vanillin assay for catechins and proanthocyanidins.J Agric Food Chem1998; 46: 4267-4274.
[16] Hanato T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effect.Chem Pharmacol1988; 36: 1090-1097.
[17] Oyaizu M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction.Jpn J Nutr1986; 44: 307-315.
[18] Jilani G, Saxena RC. Repellent and feeding deterrent effects of turmeric oil, sweetllag oil. neem oil and a neem-based insecticide against lesser grain borer (Coleoptera: Bostrychidae).J Econ Entornol1990; 83: 629-634.
[19] Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils from seven aromatics plants grown in Colombia againstSitophilus zeamaisMotschulsky (Coleoptera).J Stored Prod Res2009; 45: 212-214.
[20] McDonald LL, Guy RH, Speirs RD.Preliminary evaluation of new candidate materials as toxicants, repellents and attractants against stored-product insects. Agricultural Research Service, US Department of Agriculture. Report number: 882, 1970.
[21] Finney DJ.Probit analysis. 3 rd edition. London: Cambridge University;1971, p. 261.
[22] Statsoft. Statistica for Windows (Computer program electronic 703 manual);Statsoft, Inc.: Tulsa, OK; 1998.
[23] Khadraoui A, Khelifa A, Boutoumi H, Mettai B, Karzazi Y, Hammouti B.Corrosion inhibition of carbon steel in hydrochloric acid solution byMentha pulegiumextract.Port Electrochim Acta2014; 32(4): 271-280.
[24] Iloki-Assanga SB, Lewis-Luján LM, Lara-Espinoza CL, Gil-Salido AA, Fernandez-Angulo D, Rubio-Pino JL, et al. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis ofBucida bucerasL. andPhoradendron californicum.BMC Res Notes2015; 8: 396.
[25] Felhi S, Daoud A, Hajlaoui H, Mnafgui K, Gharsallah N, Kadri A. Solvent extraction effects on phytochemical constituents profiles, antioxidant and antimicrobial activities and functional group analysis ofEcballium elateriumseeds and peels fruits.Food Sci Technol2017; 37(3): 483-492.
[26] Sarikurkcu C, Feryat E, Mustafa C, Bektas T, Ahmet C, Ebru M. Screening of the antioxidant activity of the essential oil and methanol extract ofMentha pulegiumL. from Turkey.Inter J Rapid Commn2012; 45: 352-358.
[27] Tsibranska I, Tylkowski B. Solid-liquid extraction of bioactive compounds:Effect of polydispersity and particle size evolution.Chem Technol Metallurgy2016; 51(5): 489-499.
[28] Karray-Bouraoui N, Ksouri R, Falleh H, Rabhi M, Jaleel CA, Grignon C, et al. Effects of environment and development stage on phenolic content and antioxidant activities ofMentha pulegiumL.J Food Biochem2010; 34: 79-89.
[29] Ben Ahmed Z, Yous fi M, Viaene J, Dejaegher B, Demeyer K, Debby M,et al. Seasonal, gender and regional variations in total phenolic, flavonoid,and condensed tannins contents and in antioxidant properties fromPistacia atlanticassp. leaves.Pharm Biol2017; 55(1): 1185-1194.
[30] Khennouf S, Benchiekh D, Djidel S, Dahamna S, Amira S, Charef N, et al.Polyphenols and antioxidant properties of extracts fromMentha pulegiumL. andMatricaria chamomillaL.Phcog Commn2013; 3(2): 35-40.
[31] Benabdallah A, Rahmoune C, Boumendjel M, Aissi O, Messaoud C.Total phenolic content and antioxidant activity of six wildMenthaspecies(Lamiaceae) from northeast of Algeria.Asian Pac J Trop Biomed2016; 6(9):760-766.
[32] Shahmohamadi R, Sariri R, Rasa M, Aghamali M. Antioxidant activity of gilanMentha pulegiumduring growth.Pak J Biol Sci2014; 17: 380-387.
[33] Tomson L, Kruma Z. Influence of harvest time on the phenolic content of horseradish leaves.Foodbalt2017: 45-50. Doi: 10.22616/foodbalt.2017.019.
[34] Hamrouni Sellami I, Maamouri E, Chahed T, Aidi Wannes W, Marzouk B,Kchouk ME. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majoranaL.).Ind Crops Prod2009; 30(3): 395-402.
[35] Usano-Alemany J, Panjai L. Effects of increasing doses of UV-B on main phenolic acids content, antioxidant activity and estimated biomass in Lavandin (Lavandula × intermedia).Nat Prod Commun2015; 10(7): 1269-1272.
[36] Saeb K, Gholamrezaee S, Asadi M. Variation of antioxidant activity ofMelissa officinalisleaves extracts during the different stages of plant growth.Biomed Pharmacol J2011; 4(2): 237-243.
[37] Herms DA, Mattson WJ. The dilemma of plants: To grow or defend.Q Rev Biol1992; 67: 283-335.
[38] Aires A, Marrinhas E, Carvalho R, Dias C, Saavedra MJ. Phytochemical composition and antibacterial activity of hydroalcoholic extracts ofPterospartum tridentatumandMentha pulegiumagainstStaphylococcus aureusisolates.Biomed Res Int2016; 2016(2): 1-11.
[39] Zhou X, Wang F, Zhou R, Xie M. Apigenin: A current review on its beneficial biological activities.J Food Biochem2017; 41: e12376.
[40] Ju SM, Kang JG, Bae JS, Pae HO, Lyu YS, Jeon BH. The flavonoid apigenin ameliorates cisplatin-induced nephrotoxicity through reduction of p53 activation and promotion of PI3K/Akt pathway in human renal proximal tubular epithelial cells.Evid Based Complement Alternat Med2015;2015: 186-436.
[41] Razzaghi-Asl N, Garrido J, Khazraei H, Borges F, Firuzi O. Antioxidant properties of hydroxycinnamic acids: A review of structure-activity relationships.Curr Med Chem2013; 20(36): 4436-4450.
[42] Nickavar B, Alinaghi AM, Kamalinejad M. Evaluation of the antioxidant properties of fiveMenthaspecies.Iranian J Pharm Res2008; 7: 203-209.
[43] Khaled-Khodja N, Boulekbache-Makhlouf L, Madani K. Phytochemical screening of antioxidant and antibacterial activities of methanolic extracts of someLamiaceae.Ind Crops Prod2014; 61: 41-48.
[44] Ghasemzadeh A, Ghasemzadeh N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human.J Med Plants Res2011; 5(31):6697-6703.
[45] Gru?ová D, Labun P, ?er?eň F, ?alamon I. Seasonal variation in DPPH scavenging activity ofMentha piperita.Adv Environ Biol2012; 6(4): 1477-1480.
[46] Wang BN, Huang Q, Venkitas C, Pan Z. Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujubaMiller) during three edible maturity stages.LWT-Food Sci Technol2016; 66: 56-62.
[47] Gan J, Feng Y, He Z, Li X, Zhang H. Correlations between antioxidant activity and alkaloids and phenols of Maca (Lepidium meyenii).J food Quality2017; 2017: 1-10.
[48] De Oliveira AMF, Pinheiro LS, Pereira CKS, Matias WN, Gomes RA,Chaves OS, et al. Total phenolic content and antioxidant activity of some malvaceae family species.Antioxidants2012; 1: 33-43.
[49] Piluzza G, Bullitta S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area.Pharm Biol2011; 49(3): 240-247.
[50] Mangalat S, Narayanan V, Janardhanan M. Herbal larvicides to control mosquito larvae: A preliminary study.Nat Prod Rad2004; 3(1): 24-26.
[51] Pascual-Villalobos MJ, Robledo A. Screening for anti-insect activity in Mediterranean plants.Ind Crops Prod1998; 8: 183-194.
[52] Regnault-Roger C, Hamraoui A, Holeman M, Theron E, Pinel R.Insecticidal effect of essential oils from mediterranean plants uponAcanthoscelides obtectusSay (Coleoptera, Bruchidae), a pest of kidney bean(Phaseolus vulgarisL.).J Chem Ecol1993; 19(6): 1233-1244.
[53] Kumar P, Mishra S, Malik A, Satya S. Repellent, larvicidal and pupicidal properties of essential oils and their formulations against the housefly,Musca domestica.Med Vet Entomol2011; 25: 302-310.
[54] Pumnuan J, Khurnpoon L, Insung A. Effects of insecticidal essential oil fumigations on physiological changes in cutDendrobium soniaorchid flower.Songklanakarin J Sci Technol2015; 37(5): 523-531.
 Asian Pacific Journal of Tropical Biomedicine2018年4期
Asian Pacific Journal of Tropical Biomedicine2018年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Comparison of antioxidant capacity and α-glucosidase inhibitory activity between bitter melon (Momordica charanti) fruit and leaf extract
- Anti-inflammatory and antinociceptive activities of Rhipicephalus microplus saliva
- Physicochemical properties, antioxidant and anti-inflammatory activities of coumarin-carbonodithioate hybrids
- Efficacies of four plant essential oils as larvicide, pupicide and oviposition deterrent agents against dengue fever mosquito, Aedes aegypti Linn. (Diptera: Culicidae)
