Effective Aeromonas specific monoclonal antibody for immunodiagnosis
Yuvadee Mahakunkijcharoen, Chakrit Hirunpetcharat, Sunisa Malijunbua, Watcharamat Muangkaew, Suporn Paksanont
1Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Thailand
2Department of Microbiology, Faculty of Public Health, Mahidol University, Thailand
3Department of Medical Technology, Faculty of Science and Technology, Bansomdejchaopraya Rajabhat University, Thailand
1. Introduction
Aeromonasspecies are facultative anaerobic Gram-negative rod-shaped bacteria in the family Aeromonadaceae[1,2]. Their natural habitat is in the aquatic environment, including ground water, surface water, estuarine and marine water, treated waste water[3-5], and some certain strains ofAeromonascan also be found in chlorine-treated municipal drinking water supplies[6,7].Apart from aquatic environment, the bacteria can be found in soil and foodstuffs, including seafood, raw meats and dairy products[1,4,8,9]. As wide spread ofAeromonasin the environment is surrounding humans and animals, the bacteria are recognized as the potential pathogenic agents causing food and/or water borne infections[1,3,4]. TheAeromonasinfectious diseases can be both gastroenteritis and non-gastroenteritis[10-12]. The more serious illness is the non-gastroenteritis including skin and soft-tissue infections[13,14], septicemia[1,15,16], peritonitis[17] and various internal organ infections in humans and animals[10,18,19]. Recently the frequency ofAeromonasinfections increase and associate with high fatality, especially in immunocompromised patients[10,11].Therefore the rapid diagnosis is urgently needed, since routine diagnosis for the disease is mainly the conventional culture methods, which are both time-consuming and labor-intensive.Although modern molecular biological techniques have been used to diagnoseAeromonasinfections[3,5,9,20], they are costly and complex.Therefore, immunological techniques are considered to be more useful. These techniques require a specific antibody that recognizes a specific antigen expressed by the organism of interest. Monoclonal antibodies (MAbs) are commonly used for this purpose because they can be designed to recognize a specific epitope of an organism and can be produced with the same specificity in unlimited amounts.In this study, we produced a MAb, designated 88F2-3F4, from the splenocytes of a mouse immunized withAeromonas hydrophila(A.hydrophila) ATCC 7965. This MAb specifically bound an 8.5 kDa molecule in all 123Aeromonasisolates tested in this study. Therefore,it has potential utility as a diagnostic tool.
2. Materials and methods
2.1. Bacterial strains
One hundred twenty-threeAeromonasisolates were used in this study: 27A. hydrophilaisolates, 26Aeromonas sobria(A. sobria)isolates, 26Aeromonas caviae(A. caviae) isolates, 26Aeromonas trota(A. trota) isolates, 8Aeromonas jandaei(A. jandaei) isolates, 7Aeromonas veronii(A. veronii) isolates, and 3Aeromonas media(A.media) isolates. The phenospecies of each isolate had previously been confirmed according to their biochemical properties at the Bamrasnaradura Infectious Disease Institute, Department of Disease Control, Ministry of Public Health, Thailand.
Other Gram-negative bacteria were used as controls:Plesiomonas shigelloides(ATCC 14029),Vibrio cholerae(V. cholera) O17SR,(9701),V. choleraeO139,V. choleraenon O1/non O139(DMST2873),Salmonella enteritidis, enterohemorrhagicEscherichia coli(E. coli),Proteus vulgaris,Pseudomonas aeruginosa(ATCC 28763),Enterobacter cloacae,Klebsiella pneumoniae, andShigella flexneri1a.
The bacterial stock cultures were cryopreserved in 30% (v/v)glycerol (Bio Basic Inc., Markharn, Ontario, Canada) and 1% (w/v)peptone (Difco, Becton Dickinson, Sparks, USA), and stored at --80℃ until analysis.
2.2. Preparation of crude bacterial extracts
Bacteria from the frozen stocks were grown on tryptic soy agar plates at 37 ℃ for 18–24 h. One loopful of bacterial colonies was transferred into tryptic soy broth (TSB) containing 0.6% yeast extract and incubated in a shaking incubator (Scientific Series 25D,New Brunswick, NJ, USA) at 120 rpm for 4 h at 37 ℃. The cells were harvested by centrifugation (Beckman Coulter, Miami, FL,USA) at 20 000 rpm for 20 min at 4 ℃. The pellet was washed and centrifuged once with sterile phosphate-buffered saline (PBS; pH 7.4). The cells in the pellet were then lysed by sonication (Vibra-Cell?, Sonics&Materials, Danbury, CT, USA), and the bacterial debris were removed by centrifugation. The supernatant was collected and stored in aliquots at -20 ℃ until analysis. The dry weights of the crude bacterial extracts were determined.
2.3. Production of Aeromonas-specific monoclones
2.3.1. Immunization
Inbred BALB/c female mice, aged 6–8 weeks, with a mean weight of 25 g, were purchased from the National Laboratory Animal Center, Mahidol University. The mice were subcutaneously inoculated three times at two-week intervals with 100 μL of 2 mg/mL whole cell extract fromA. hydrophilaATCC 7965 mixed with Montanide ISA 720 (SEPPIC SA, Paris, France) adjuvant. The titers of antibodies specific forA. hydrophilaATCC 7965 in the immunized mouse sera were determined with an indirect enzyme-linked immunosorbent assay (indirect ELISA). The study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University (MUTM 2008-009-01).
2.3.2. Hybridoma technology
Monoclones were produced from hybridoma technology modified from Kohler and Milstein[21]. Splenocytes from immunized mice with serum antibody titers>204 000 were mixed with PS-X63-Ag8 myeloma cells (kindly provided by Professor Watchara Kasinrerk,Chiang Mai University, Thailand) in a ratio of 10: 1 in the presence of polyethyleneglycol (PEG, Sigma) for 90 s. The polyethyleneglycol was then removed by centrifugation. The cell mixture was suspended in Dulbecco’s modified Eagle’s medium containing hypoxanthine,aminoptherin, and thymidine (HAT medium) and 10% fetal bovine serum, and then distributed into the wells of a 96-well flat-bottom microculture plate (Corning Inc., Mexico) and incubated at 37 ℃in a 5% CO2atmosphere. The growing hybridomas were observed under an inverted microscope.
2.3.3. Screening for Aeromonas-specific antibody-producing hybridomas
The supernatants from the growing hybridomas were tested for antibodies specific for antigens ofA. hydrophilaATCC 7965 with an indirect ELISA (modified from Engvall and Perlmann)[22]. Briefly,100 μL of crude bacterial extract (20 μg/mL) in coating buffer(carbonate/bicarbonate buffer, pH 9.6) was added to the individual wells of a 96-well microtiter plate (Greiner, Frickenhausen,Germany) and incubated in a moist chamber at 4 ℃ for 18–24 h. The plates were washed three times with washing buffer [PBS+0.05%Tween 20 (PBST)]. The unoccupied space in each well was filled with 200 μL/well blocking solution [3% skim milk (Difco, Becton Dickinson) in PBST], and the plates were incubated in a moist chamber at 37 ℃ for 1 h. After the blocking solution was removed,the wells were filled with 100 μL of two-fold serially diluted mouse immune serum, normal mouse serum (to determineAeromonasspecific mouse antibody titer), or hybridoma/monoclone culture supernatant as a test sample, myeloma culture supernatant as the negative control, or diluent only as the blank control (to screen for clones producingAeromonas-specific antibody). The plates were incubated at 37 ℃ for 1 h. The unbound antibodies were removed with four washes with PBST. Horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG)+IgM+IgA(Invitrogen, Carlsbad, CA, USA), diluted 1: 5 000 in 1% skim milk/PBST (100 μL/well), was added and incubated at 37 ℃ for 1 h.After four washes (as described above), soluble substrates [1 mg/mLo-phenylenediamine dihydrochloride in citrate–phosphate buffer (pH 5.0) and 0.03% (v/v) H2O2] were added to the plates, and kept in the dark at room temperature for 30 min. The reaction was stopped with 2.5 N H2SO4(50 μL/well). The reaction strength was determined by measuring the optical density at 492 nm (OD492) with a microplate reader (Tecan, Austria).
The antibody producing hybridomas were expanded and their culture supernatants were collected to test their antibody-binding activities with the otherAeromonasspecies and other Gram-negative bacteria listed above. The hybridomas that produced antibodies specific for eitherA. hydrophilaonly or for all theAeromonasspecies,but did not bind other Gram-negative bacteria, were selected.Monoclones were obtained from the selected hybridomas with limiting dilution. The supernatants collected from the monoclone cultures were screened again forAeromonasspecificity. The positiveAeromonas-specific antibody-producing monoclones were maintained in Dulbecco’s modified Eagle’s medium containing 10%fetal bovine serum and also preserved in liquid nitrogen. The culture supernatants were collected for further characterization.
2.3.4. Characterization of Aeromonas-specific monoclonal antibody
TheAeromonasantigen specific to each MAb was characterized with a Western blotting analysis. First, antigens (10 μg/lane) from all the tested bacteria were fractionated with sodium dodecyl sulfatepolyacrylamide gel electrophoresis using 10% (w/v) polyacrylamide or 5%–20% gradient gel (e-PAGEL, ATTO Corporation, Tokyo,Japan) and a constant current of 20 mA/gel. After electrophoresis,the fractionated proteins on the polyacrylamide gel were transferred to a prewetted polyvinylidene fluoride (PVDF) membrane with a 0.2 μm pore size (PALL Life Sciences) with an electroblotting unit[Hoefer Semiwet, Amersham Bioscience (SF) Corp., USA] at a constant voltage of 25 V for 4.5 h. The blotted PVDF membranes were then blocked with 3% skim milk/PBS for at least 1 h. The membranes were then allowed to react with the culture supernatants and/or myeloma culture supernatant (as the negative control) at room temperature. After 2 h, the reacted membranes were washed with PBST (about 3–4 changes in 3–5 min), and incubated with HRP-conjugated goat anti-mouse IgG+IgA+IgM (Invitrogen,Carlsbad, CA, USA), diluted 1: 5 000 in 1% skim milk/PBS, at room temperature for 1 h. After the membranes were washed with PBST,they were incubated with DAB substrate solution [1 mg/mL 3,3’-diaminobenzidine in 0.1 M Tris-HCl (pH 7.4) and 0.03% (v/v) H2O2]in the dark at room temperature for 5 min. The reaction was stopped by washing the membranes with distilled water.
The molecular masses of the reactive bands were determined by comparing their relative mobilities against a standard curve constructed from the molecular masses of standard protein markers run on the same gel. The cross-reactions between these MAbs and other related crude Gram-negative bacterial antigens were also tested.
2.3.5. Identification of immunoglobulin isotype and subclass by indirect ELISA
The isotype and/or subclass of the MAb were identified with an ELISA using the Mouse MonoAb ID Kit (HRP) from Zymed Laboratories Inc. (South San Francisco, CA, USA). Briefly, the immunoglobulins in monoclone culture supernatants from the monoclone 88F2-3F4 culture were added into a 96-well flat-bottom microplates (Greiner, Frickenhausen, Germany) coated with the crude antigens ofA. hydrophilaATCC 7965. After incubation for 2 h, the binding immunoglobulin was allow to react with rabbit IgG specific for mouse μ, γ1, γ2a, γ2b, γ3, α chain and κ or λL chain. Goat anti-rabbit IgG conjugated with horseradish peroxidase was then added and the reaction was visualized by the enzymatic reaction between HRP and 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid).
2.3.6. Determination of monoclonal antibody strength
The sensitivity of the MAb was determined with a dot-blot ELISA.CrudeAeromonaslysate was two-fold serially diluted and dotted onto pieces of PVDF membrane. After the membrane was air dried, it was reacted with the monoclone culture supernatants, as an indirect ELISA.
2.4. Efficacy of MAb determined by dot blot ELISA
One hundred twenty-six rectal swabs in Stuart’s transport medium were obtained from the Bamrasnaradura Infectious Diseases Institute,Thailand. The use of these samples as expedited samples was approved by the Institutional Review Board of the Faculty of Tropical Medicine. All rectal swabs were transferred into tubes containing 2 mL of TSB and incubated overnight at 35 ℃. The TSB cultures were stored as aliquots at --75 ℃ until analysis. Of these 126 samples, none were positive forAeromonaswith conventional testing. Therefore, various concentrations ofA. hydrophilaATCC 7965 were blindly seeded into 37 rectal swab cultures and 5 μL of each sample was dotted onto PVDF membrane. TheAeromonasantigens presented in the cultures were detected with the dot-blot ELISA. The procedure of the test was similar to indirect ELISA mentioned above.
The correlation between the results of dot-blot ELISA and the knownAeromonasseeded samples was statistical analyzed by the calculation of Kappa coefficience (κ)[23,24].
3. Results
3.1. Monoclonal antibody production
Among the several hundred of hybridomas obtained from one fusion of splenocytes from a mouse immunized withA. hydrophilaATCC 7965 and P3-X63-Ag8 myeloma cells, hybridoma 88F2 was selected as its production of an antibody that reacted strongly to antigens extracted from all theAeromonasstrains tested. This hybridoma was subcloned until the monoclone designated 88F2-3F4 was obtained.
3.2. Determination of monoclonal antibody isotype
The immunoglobulin produced from monoclone 88F2-3F4 showed high reaction to rabbit anti-mouse γ2a and κ L chain determined by ELISA, therefore its isotype should be IgG2a, κL chain (Figure 1).
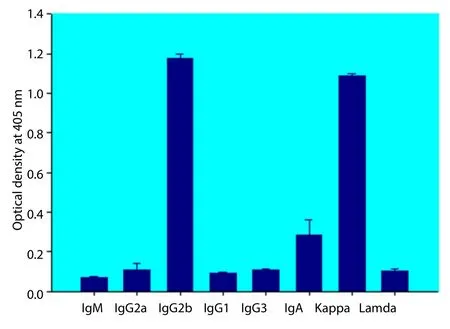
Figure 1. Identification of immunoglobulin isotype of MAb 88F2-3F4 with an indirect ELISA.
3.3. Specificity of MAb 88F2-3F4
In an indirect ELISA, MAb 88F2-3F4 reacted strongly with 42 of 44 crudeAeromonaslysates prepared from sevenAeromonasspecies and reacted weakly with the remaining 2 isolates (OD492of the reactions varied from 0.106 to 1.411) (Figures 2A–2G), indicating that the MAb recognizes a common antigen in allAeromonasspp.In dot-blot ELISAs using the crude antigens produced from 123 isolates of sevenAeromonasspecies, the MAb reacted with all theAeromonasisolates (Figure 3).
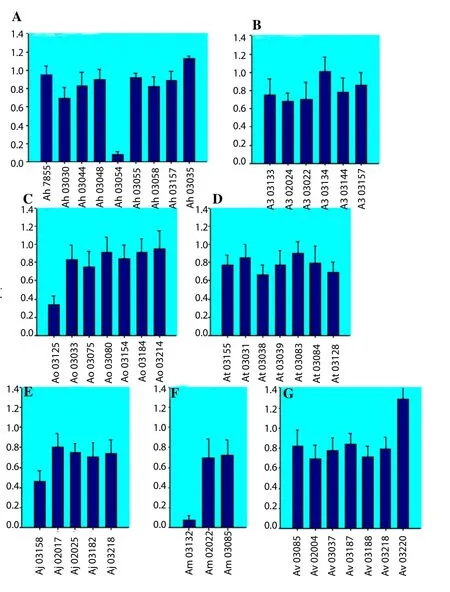
Figure 2. Specificity of MAb 88F2-3F4.
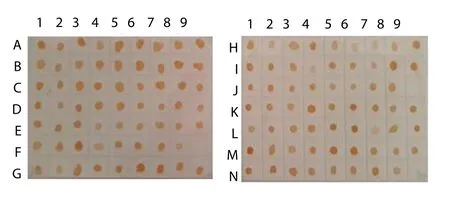
Figure 3. MAb 88F2-3F4 specificity.
To examine the cross-reactivity of the MAb with other Gramnegative bacteria, it was tested against the crude lysates of 11 different Gram-negative bacteria:V. choleraeO1 (Vc O17SR),V. choleraeO139,V. choleraenon O1/non O139,Plesiomonas shigelloides,E. coli,Salmonella typhimurium,Shigella flexneri,Enterobacter cloacae,Klebsiella pneumoniae, andProteus vulgaris. The MAb did not cross-react with these bacteria (Figure 4).
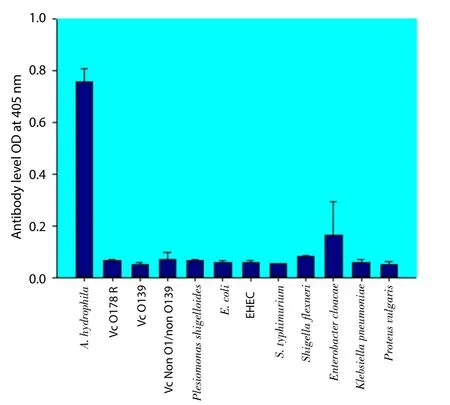
Figure 4. Specificity of MAb 88F2-3F4.
The antigen was recognized by the MAb using Western blotting.Seven crudeAeromonaslysates were fractionated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 5%–20%gradient polyacrylamide gel, then blotted onto PVDF membrane and reacted with the MAb. The MAb reacted with a band of relative molecular mass (Mr) ~8.5 kDa in the lysates of all sevenAeromonasspecies, but did not react with the lysates of the other 11 Gram-negative bacteria (Figure 5). We concluded that the specificAeromonasantigen recognized by MAb 88F2-3F4 has a relative molecular mass of about 8.5 kDa.
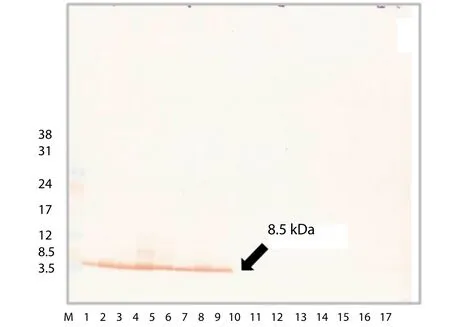
Figure 5. MAb 88F2-3F4 specificity.
3.4. Sensitivity of MAb 88F2-3F4
To determine the sensitivity of MAb 88F2-3F4 in detecting theAeromonasantigen, a crude bacterial extract ofA. hydrophilaATCC 7965 was two-fold serially diluted (from 250 to 0.49 μg/mL), dotted onto PVDF membrane, and reacted with the MAb. MAb 88F2-3F4 reacted with the antigen at a concentration of 3.9 μg/mL, indicating the modest sensitivity of this MAb (Figure 6).
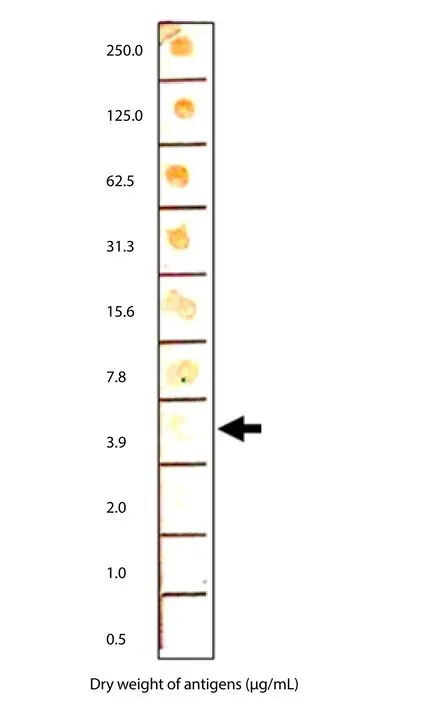
Figure 6. MAb 88F2-3F4 sensitivity.
3.5. Efficiency of MAb 88F2-3F4
The efficiency of MAb 88F2-3F4 in detecting theAeromonasantigen was evaluated using artificially seeded rectal swab samples.Thirty seven of one hundred twenty-sixAeromonasnegative rectal swab cultures were blindly seeded with various concentrations of crude antigens fromA. hydrophilaATCC 7965. An aliquot (5 μL) of each seeded sample was dotted onto PVDF membrane (PALL Life Sciences) and reacted with MAb 88F2-3F4. Of the 37Aeromonasantigen-seeded rectal swab samples, 32 samples (86.49%) were positive after detecting with MAb 88F2-3F4 and 5 samples were definitely negative. In the number of 89 unseeded samples, 82 samples were truly negative and 7 samples showed mildly positive.These results indicate that MAb 88F2-3F4 has 86.49% sensitivity,92.13% specificity, 92.68% accuracy, 82.05% positive predictive value and 94.25% negative predictive value. The dot blot ELISA using MAb 88F2-3F4 could detectAeromonasantigens in the artificial seeded rectal swab cultures with high correlation to the known number of theAeromonasseeded samples as κ value was 0.774.
4. Discussion
Aeromonasspp. survive at a wide range of temperatures, pHs,salt concentrations, and chlorine concentrations[1,7,25]. They occur throughout the environment, especially in water and soil, and are one of the indicators of fecal and waste contamination[26].Aeromonasspp.produces hemolysin and enterotoxin, asV. choleraandSalmonellaspp. Therefore, the bacteria are recognized as pathogens that cause diseases in humans and animals. Their prevalence in humans has not been extensively studied, but according to our observations based on the collection of rectal swabs from a laboratory service, it is about 3%.Aeromonascan infect wounds and induce serious clinical problems, including septicemia and the infection of various internal organs. In some cases of infection in immunocompromised hosts,it has more serious consequences. Therefore, sensitive diagnostic tests for this infection may have utility in the surveillance of this reemerging disease and consequently its prevention.
Although the technology of MAb production has been established for more than 35 years, the broad application of MAbs is still increasing. The production of MAbs directed againstAeromonasspp. has been achieved by many investigators, including Delamareet al.[27]. They used splenocytes from a mouse immunized withA.hydrophilaATCC 7966 and obtained MAb 5F3 with specificity for a 110 kDa antigen on the whole cells ofA. hydrophila. The specificity of MAB 5F3 was determined with an ELISA. The researchers reported that the MAb reacted strongly only withA. hydrophilabut produced a basal reaction toA. sobria,A. trota,Aeromonas salmonicida,Aeromonas enteropelogenes,Aeromonas ichtiosmia, and another five Gram-negative bacteria:Salmonella typhimurium,E.coli,Enterobacter cloacae,Pseudomonas aeruginosa, andPseudomonas putida. The sensitivity of MAb 5F3 for detection ofA. hydrophilawas 105cell/mL[27].
In 2007, Longyant and colleagues[28] also producedAeromonasspecific MAbs, which were classified into three groups according to their recognition of differentAeromonasantigens. MAbs in Group 1 reacted with 10–200 kDaAeromonasantigens. The MAbs in Group 2 reacted with 10–200 kDa antigens from two of the threeA. hydrophilastrains tested in the study. The MAbs in Group 3 reacted with all theAeromonasstrains tested in the study,i.e., threeA. hydrophila, twoA. sobria, and oneA. caviae. However, its reaction withA. caviaewas weak and only sixAeromonasisolates were tested in the study. The sensitivities of these three MAbs, determined with dot-blot ELISAs, were about (106–107) cfu/mL.
In the present study, we produced theAeromonas-specific MAb 88F2-3F4. This antibody reacted with all 123 isolates of sevenAeromonasspecies tested, and did not cross-react with other Gramnegative bacteria typically found in the gastrointestinal tract.Therefore, MAb 88F2-3F4 is anAeromonas-specific MAb and can be used as an immunodiagnostic tool for the detection ofAeromonasinfection or contamination in clinical or environmental samples.
On a dot-blot ELISA, MAb 88F2-3F4 detected concentrations of crudeAeromonasantigen as low as 3.9 μg/mL. The efficacy of the MAb may be rather low because the specific antigen with which it reacts is very small (8.5 kDa).
When this MAb was used to detect theAeromonasantigen in artificial seeded rectal swab cultures, the sensitivity, specificity, and accuracy of MAb 88F2-3F4 were 86.49%, 92.13%, and 92.68%,respectively. Therefore, this MAb may be useful for the rapid detection ofAeromonasin clinical samples from both humans and animals and for screening bacterial contamination of foodstuffs,waste, and the environment.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We gratefully acknowledge Mr. Boonchuay Eampokalap and the staff of the Microbiology Section, Bamrasnaradura Infectious Disease Institute, Department of Disease Control, Ministry of Public Health, for kindly providingAeromonasfrom clinical isolates and rectal swabs that were available after routine diagnoses. We also express our sincere thanks to Professor Watchara Kasinrerk of Chiang Mai University for his generous gift of the PS-X63-Ag8 myeloma cells.
Funding
This work was supported by Grant No.59/2549 from Mahidol University and Grant No. 04/2554 from the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
[1] Janda JM, Abbott SL. The genusAeromonas: taxonomy, pathogenicity,and infection.Clin Microbiol Rev2010; 23: 35-73.
[2] Talagrand-Reboul E, Jumas-Bilak E, Lamy B. The social life ofAeromonasthrough biofilm and quorum sensing systems.Front Microbiol2017; 8: 1-19.
[3] Olaniran AO, Nzimande SBT, Mkize NG. Antimicrobial resistance and virulence signatures of Listeria andAeromonasspecies recovered from treated wastewater effluent and receiving surface water in Durban, South Africa.BMC Microbiol2015; 15: 234-243.
[4] Tomás J. The mainAeromonaspathogenic factors.ISRN Microbiol2012;256261: 1-22.
[5] Igbinosa IH, Okoh AI. Detection and distribution of putative virulence associated genes inAeromonasspecies from freshwater and wastewater treatment plant.J Basic Microb2013; 53: 895-901.
[6] Holmes P, Nicolls L. Aeromonads in drinking-water supplies: their occurrence and significance.J Chart Inst Water Environ Manage1995; 9:464-469.
[7] Messa S, Armuzzi R, Tosques M, Cangane F, Trovatelli LD. Susceptibility to chlorine ofAeromonas hydrophilastrains.J Appl Microbiol1999; 86:169-173.
[8] Martins L, Marquez R, Yano T. Incidence of toxicAeromonasisolated from food and human infection.FEMS Immunol MedMicrobiol2002; 32:237-242.
[9] Nagar V, Shashidhar R, Bandekar JR. Characterization ofAeromonasstrains isolated from Indian foods using rpoD gene sequencing and whole cell protein analysis.World J Microbiol Biotechnol2013; 29: 745-752.
[10] Parker J, Shaw J.Aeromonasspp. clinical microbiology and disease.J Infect2011; 62: 109-111.
[11] Rhee JY, Jung DS, Peck KR. Clinical and therapeutic implications ofAeromonas bacteremia: 14 years nation-wide experiences in Korea.Infect Chemother2016; 48: 274-284.
[12] Daskalov H. The importance ofAeromonas hydrophilain food safety.Food Control2006; 17: 474-483.
[13] Park SY, Woong-Kyo J, Kim MJ, Lee KM, Lee WS, Lee DH. Necrotising fasciitis in both calves caused byAeromonas caviaefollowing aesthetic liposuction.Plast Reconstr Surg2010; 63: e695-e698.
[14] Spadaro S, Berselli A, Marangoni E, Romanello A, Colamussi MV,Ragazzi R, et al.Aeromonas sobrianecrotizing fasciitis and sepsis in an immunocompromised patient: a case report and review of the literature.J Med Case Rep2014; 8: 1-6.
[15] Janda JM, Guthertz LS, Kokka RP, Shimad T.Aeromonasspecies in septicemia: laboratory characteristics and clinical observations.Clin Infect Dis1994; 19: 77-83.
[16] Wu CJ, Chen PL, Hsueh PR, Chang MC, Tsai PJ, Shih HI, et al. Clinical implications of species identification in monomicrobialAeromonas bacteremia.PloS One2015; 10: e0117821. Doi:10.1371/journal.pone.0117821.
[17] Choi JP, Lee SO, Kwon HH, Kwak YG, Choi SH, Lim SK, et al. Clinical significance of spontaneousAeromonasbacterial peritonitis in cirrhotic patients: a matched case-control study.Clin Infect Dis2008; 47: 66-72.
[18] Rey A, Verján N, Ferguson HW, Iregui C. Pathogenesis ofAeromonas hydrophilastrain KJ99 infection and its extracellular products in two species of fish.Vet Rec2009; 164: 493-499.
[19] Praveen P, Debnath C, Shekhar S, Dalai N, Ganguly S. Incidence ofAeromonasspp. infection in fish and chicken meat and its related public health hazards: a review.Vet World2016; 9: 6-11.
[20] Delamare APL, Echeverrigaray S, Duarte KR, Gomes LH, Costa SOP.Total protein electrophoresis and RAPD fingerprinting analysis for the identification ofAeromonasat the species level.Brazil J Microbiol2002;33: 358-362.
[21] Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity.Nature1975; 256: 495-497.
[22] Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA).Quantitative assay of immunoglobulin G.Immunochemistry1971; 8: 871-874.
[23] Landis J, Koch G. The measurement of observer agreement for categorical data.Biometrics1977; 33: 159-174.
[24] Cohen J. A coefficiency of agreement for nominal scales.Educ Psychol Meas1960; 20: 37-46.
[25] Martin-Carnahan A, Joseph SW. Aeromonas. In:Bergeys’manual of systematic bacteriology. Vol 2, Part B. Brenner DJ, Krieg NR, Staley JT,editors. New York: Williams and Wilkins; 2005, p. 556-578.
[26] Percival S, Williams D. Aeromonas. In:Microbiology of waterborne diseases. 2nd ed. San Diego: Academic Press; 2014.
[27] Delamare APL, Echeverrigaray S, Duarte KR, Gomes LH, Costa SOP.Production of a monoclonal antibody againstAeromonas hydrophilaand its application to bacterial identification.J App Microbiol2002; 92: 936-940.
[28] Longyant S, Prahkarnkaeo K, Meevoothisoon V, Rengpipat S,Rukpratanporn S, Sithigorngul W. Identification ofAeromonas hydrophilainfection with specific monoclonal antibodies.Mj Int J Sci Tech 2007; 1:107-119.
 Asian Pacific Journal of Tropical Biomedicine2018年1期
Asian Pacific Journal of Tropical Biomedicine2018年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Prevalence of coronavirus from diarrheic calves in the Republic of Korea
- Pharmacodynamic profiling of optimal sulbactam regimens against carbapenemresistant Acinetobacter baumannii for critically ill patients
- Efficiency of combining pomegranate juice with low-doses of cisplatin and taxotere on A549 human lung adenocarcinoma cells
- Correlation of phytochemical content with antioxidant potential of various sweet potato (Ipomoea batatas) in West Java, Indonesia
- Larvicidal activity of Xenorhabdus and Photorhabdus bacteria against Aedes aegypti and Aedes albopictus
- Proximate composition, nutritional values and phytochemical screening of Piper retrofractum vahl. fruits
