A new species of smooth skink(Squamata:Scincidae:Scincella)from Cambodia
Thy Neang,Somaly Chan,Nikolay A.Poyarkov,Jr.
1Wild Earth Allies,Sk.Phnom Penh Thmei,Kh.Sen Sok,Phnom Penh,Cambodia
2Ministry of Environment,Sk.Tonle Bassac,Kh.Chamkarmorn,Phnom Penh,Cambodia
3Department of Vertebrate Zoology,Biological Faculty,Lomonosov Moscow State University,Moscow 119234,Russia
4Joint Russian-Vietnamese Tropical Research and Technological Centre,Nghia Do,Cau Giay,Hanoi,Vietnam
INTRODUCTION
The family Scincidae is one of the most globally diverse groups of lizards with 146 genera and about 1 650 species currently recognized worldwide(Uetz et al.,2018).Of these,the smooth skink genusScincellaMittleman,1950 currently contains 34 species with fragmented distribution,from the North American continent( five species)to Japan,Ryukyu Archipelago and Taiwan,China,Korean Peninsula,mainland China,and Southeast Asia(remaining species)(Ouboter,1986;Uetz et al.,2018).Scincellaspecies are characterized by their small size,elongated body,short limbs,relatively long tail,smooth subcycloid scales(most species),small oblong head with transparent disc in a movable lower eyelid,absence of supranasals,pentadactyl hindlimbs,one row of basal subdigital lamellae(most species),median preanals overlapping lateral ones,four or more scales bordering the parietals between the upper secondary temporals,and lower secondary temporal overlapping the upper one(diagnosis follows Greer&Shea,2003;Lim,1998;Nguyen et al.,2010a,2010b,2010c).Furthermore,the genusScincellais differentiated from closely relatedSphenomorphusFitzinger by the presence of a transparent window in the lower eyelid as opposed to lower eyelid covered with polygonal scales inSphenomorphus(Greer,1974;Nguyen et al.,2010a).
The phylogenetic relationships ofScincellaand many other Southeast Asian lygosomine skinks remain unresolved because they share many morphological similarities(e.g.,Nguyen et al.,2010a,2010b). Based on examination of museum specimens,Ouboter(1986)undertook a major revision ofScincellaMittleman,1950,which resulted in numerous synonymies,some of which are discussed in the present paper(see Discussion). The morphological similarities and taxonomic uncertainty have hampered further progress in the systematics of smooth skinks,with only a few species described in the last 15 years,including three taxa discovered from Vietnam(Darevsky et al.,2004;Nguyen et al.,2010a,2010b)and one from Mexico(García-Vázquez et al.,2010).In the present paper,we follow the taxonomy proposed by Darevsky(1990),who transferredSphenomorphus rufocaudatusDarevsky&Nguyen,1983 to the genusScincellaasScincella rufocaudata(Darevsky&Nguyen,1983)without providing any detailed information on this assignment.This taxonomy was accepted subsequently by Nguyen et al.(2011)and Neang&Poyarkov(2016).Scincella rufocaudatawas reported from Cambodia by Stuart et al.(2006)and Stuart&Emmett(2006)based on specimens from the Mondulkiri Province and Cardamom Mountains of southwest Cambodia(see Discussion). Therefore,to date,the genusScincellain Cambodia is represented by four species: that is,S.melanosticta(Boulenger),S.cf.rufocaudata(Darevsky&Nguyen),S.reevesii(Gray),andS.cf.rupicola(Smith)(Grismer et al.,2007,2008;Neang et al.,2010;Stuart&Emmett,2006,Stuart et al.,2006,2010)(see below forS.cf.rupicola).
Following recent changes in and transfer of the protected area management from the Ministry of Agriculture,Forestry,and Fisheries to the Ministry of Environment of Cambodia,the Keo Seima Biodiversity Conservation Area was reorganized and renamed as the Keo Seima Wildlife Sanctuary,covering an area of 292 690 hectares and spanning the Mondulkiri and Kratie provinces of south-eastern Cambodia.The sanctuary is located in the Keo Seima,O’Raing,and Senmorom districts in Mondulkiri Province and Snoul District of Kratie Province in Cambodia(Figure 1).Despite its high biodiversity,low level of disturbance,and high percentage of forest cover(Nuttall et al.,2015),little is known about the sanctuary’s herpetofauna.Recent herpetological fi eld surveys in Cambodia have focused on the Cardamom Mountains(Grismer et al.,2007,2008;Neang et al.,2010,2015;Stuart&Emmett,2006),with only two undertaken in Mondulkiri Province(Neang&Poyarkov,2016;Stuart et al.,2006).Biogeographically,the hilly areas of the eastern plain of Cambodia are linked to the Annamite Range(or Truong Son Mountains)of Vietnam(Poyarkov et al.,2017;Stuart et al.,2006),where many new herpetofaunal species have been described in recent years(Hartmann et al.,2013;Nazarov et al.,2012;Nguyen et al.,2013;Poyarkov et al.,2014,2015a,2015b;Rowley et al.,2016).
During a field survey at Prey Lang in northern central Cambodia between June and July 2014,10 specimens were collected and tentatively assigned toScincellacf.rupicolabased on their external morphology(Hayes et al.,2015;see Discussion).During a second herpetofaunal survey between 22 and 28 September 2016 in Keo Seima Wildlife Sanctuary in south-east Cambodia,we recorded nine species of amphibians and 17 species of reptiles.Among these,eight specimens were assigned to the genusScincellabased on their body habitus and external morphology.However,further morphological and molecular analyses indicated that this population represents a yet to be described species ofScincella,which we describe herein.
MATERIALS AND METHODS
Sampling
The herpetofauna field survey was undertaken during the day and night between 22 and 28 September 2015 in semi-evergreen forestin Keo Seima Wildlife Sanctuary.Specimens were captured by hand and kept in plastic bags until the next morning.Specimens were photographed prior to euthanasia and subsequent preservation in 10%formalin.Liver tissue samples were taken for molecular analyses prior to preservation in formalin and subsequently stored in 95%ethanol.Upon arrival to the collection,the specimens were washed in water for 12 h,then transferred to 70%ethanol for storage. Specimens were deposited in the Zoological Museum at the Centre for Biodiversity Conservation of the Royal University of Phnom Penh(CBC RUPP).Additionally,we examined the type series,including the holotype specimen ofSphenomorphus rufocaudatusDarevsky&Nguyen,1983(ZISP 19797,St.Petersburg,Russia).
Morphological analyses
Characters were observed under a Nikon SMZ 645 dissecting microscope and measured with a digital caliper to the nearest 0.1 mm and ratio to 0.01. The following morphometric characters were measured:eye diameter(ED)-maximum horizontal diameter of eye;forearm length(FoL)-length between forelimb elbow and tip of fourth finger with limb held at right angle to body;forelimb length(FlL)-length between axilla and tip of fourth finger with limb held at right angle to body;head depth(HD)-maximum height posterior to extremity of eye;head length(HL)-length from tip of snout to posterior margin of parietals;hind limb length(HlL)-length from groin and tip of fourth toe with limb held at right angle to body;head width(HW)-maximum width of head;snout-forelimb length(SFlL)-length from snout to anterior margin of axilla;snout length(SnL)-length from anterior corner of eye to tip of snout;snout-tympanum length(STL)-length from snout to anterior margin of tympanum;snout to vent length(SVL)-length from tip of snout to vent;tail length(TaL)-length from vent to tip of tail;tympanum diameter(TD)-maximum diameter of ear;trunk length(TrunkL)-length from posterior margin of axilla to anterior groin,with limbs held at right angles to body.Scale counts included:supralabials(SL)-number of upper labial scales;infralabials(IL)-number of lower labial scales;temporals including primary temporals-number of scales above posterior supralabial,posterior postsuboculars,and below parietal and secondary temporal;supraciliariescounted following Ouboter(1986)and Lim(1998);enlarged nuchals(EnLN)-number of enlarged nuchal scales contacting to parietals posteriorly;midbody scale rows(MBSR)-scales around midpoint of trunk;paravertebral scale rows(PVSR)-number of scales from posterior edge of parietals to point opposite vent;dorsal scale rows between dorsolateral stripes(DBR)-number of dorsal scale rows at midbody between dark dorsolateral stripes,following Inger et al.(1990);ventral scales(VS)-number of scales between gulars and preanal scales;subdigital lamellae under fourth finger(SDLF4);subdigital lamellae under fourth toe(SDLT4).
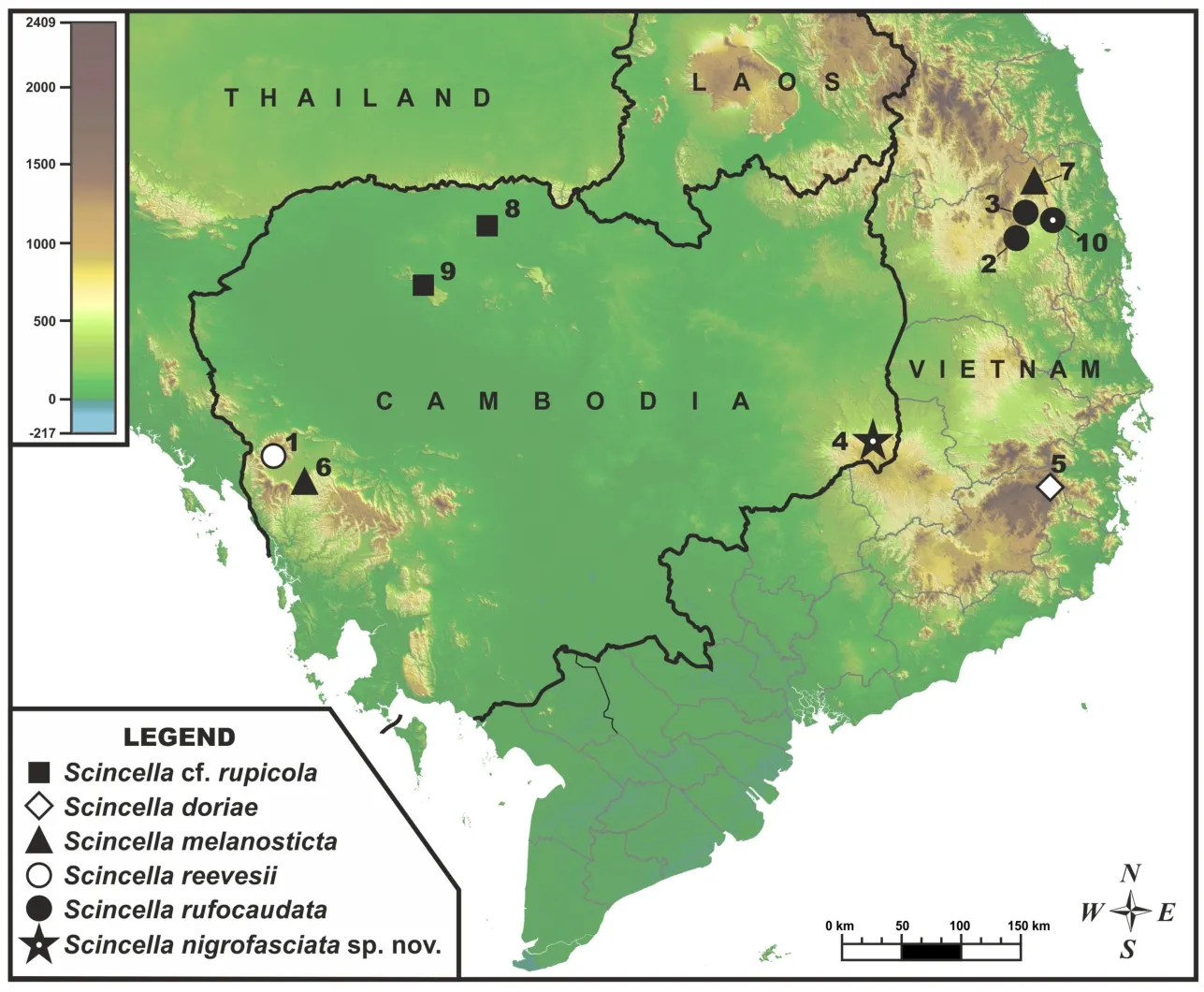
Figure 1 Map showing type locality of Scincella nigrofasciata sp.nov.at Keo Seima Wildlife Sanctuary,eastern plain,Cambodia,and locations of populations included in molecular analyses
Morphological data used for comparisons were taken from previously published literature,namely,Taylor(1963);Darevsky&Nguyen(1983);Ouboter(1986);Darevsky&Orlov(1997);Gonzalez et al.(2005);Stuart et al.(2006);Stuart&Emmett(2006);Nguyen et al.(2010a,2010b);Luu et al.(2013),and Pham et al.(2015)(Table 1),and from examination of museum specimens(Appendix I).Museum abbreviations include:CBC,Centre for Biodiversity Conservation,Royal University of Phnom Penh,Cambodia;ZISP,Zoological Institute,Russian Academy of Sciences,St. Petersburg,Russia;ZMMU,Zoological Museum of Moscow University,Moscow,Russia.
DNA isolation,PCR,and sequencing
For molecular analysis,we examined 22 specimens ofScincellafrom Cambodia and adjacent areas of Vietnam,with the sequence fromSphenomorphus maculatusused as an outgroup(Table 2).The geographic locations of the examined populations are shown in Figure 1.
For molecular phylogenetic analyses,total genomic DNA was extracted from ethanol-preserved femoral muscle and liver tissues using standard phenol-chloroform-proteinase K(final concentration 1 mg/mL)extraction,with subsequent isopropanol precipitation(protocols per Hillis et al.,1996 and Sambrook et al.,1989).Isolated total genomic DNA was visualized by 1.5%agarose gel electrophoresis in the presence of ethidium bromide.The concentration of total DNA was measured in 1μL using a NanoDrop 2000(Thermo Scientific,USA),and consequently adjusted to 100 ng DNA/μL.
We ampli fi ed a 653-bp fragment of cytochrome oxidase I(COI),a mitochondrial marker widely used as a DNA-barcoding marker for vertebrates,including reptiles and amphibians(Murphy et al.,2013;Nagy et al.,2012;Smith et al.,2008),and for species identi fi cation in various groups of lizards(Amarasinghe et al.,2017;Hartmann et al.,2013;Nazarov et al.,2012,2014;Orlova et al.,2017 Solovyeva etal.,2011,2014). Weused two primerpairsfor PCR and sequencing,depending on their performance in PCR,for different samples. The fi rst primer pair was VF1-d(5′-TTCTCAACCAACCACAARGAYATYGG-3′,forward primer)and VR1-d(5′-TAGACTTCTGGGTGGCCRAARAAY CA-3′,reverse primer)(Ivanova et al.,2006);the second primer pair was RepCOI-F(5′-TNTTMTCAACNAACCACAAA GA-3′,forward primer)and RepCOI-R(5′-ACTTCTGGRTGKC CAAARAATCA-3′,reverse primer)(Nagy et al.,2012).PCR arrays were performed in 25-μL reactions using 50 ng of genomic DNA,10 pmol of each primer,15 nmol of each dNTP,50 nmol additional MgCl2,Taq PCR buffer(10 mmol/L Tris-HCl,pH 8.3,50 mmol/L KCl,1.1 mmol/L MgCl2,and 0.01%gelatin),and 1 U of Taq DNA polymerase.The PCR conditions for theCOIgene fragment followed Nazarov et al.(2012)and included an initial denaturation step at 95°C for 3 min;5 cycles at 95°C for 30 s,annealing at 45°C for 1 min,extension at 72°C for 2 min,followed with 35 cycles at 95°C for 30 s,annealing at 51°C for 1 min,extension at 72°C for 2 min,and fi nal extension at 72°C for 5 min.
The PCR products were loaded onto 1.5%agarose gels in the presence of ethidium bromide and visualized by agarose electrophoresis. If distinct bands were produced,products were purified using 2μL from a 1:4 dilution of ExoSap-It(Amersham,UK)per 5μL of PCR product prior to cycle sequencing.The 10-μL sequencing reaction included 2 μL of template,2.5 μL of sequencing buffer,0.8 μL of 10 pmol primer,0.4μL of BigDye Terminator v3.1 Sequencing Standard(Applied Biosystems,USA),and 4.2μL of water.The cycle sequencing reaction consisted of 35 cycles of 10 s at 96°C,10 s at 50°C,and 4 min at 60°C.Cycle sequencing products were puri fi ed by ethanol precipitation.Sequence data collection and visualization were performed on an ABI 3730xl automated sequencer(Applied Biosystems,USA).The obtained fragments were sequenced in both directions for each sample,and a consensus sequence was generated using SeqMan v5.06(Burland,1999).The obtained sequences were deposited in GenBank under accession numbers MH119607-MH119629(Table 2).
Phylogenetic analyses
TheCOIdataset subjected to phylogenetic analyses included 22Scincellarepresentatives from Cambodia and Vietnam andSphenomorphus maculatusused as an outgroup toScincellabased on Pyron et al.(2013)(Table 2).
Nucleotide sequences were initially aligned using ClustalX 1.81(Thompson et al.,1997)with default parameters,and then optimized manually in BioEdit 7.0.5.2(Hall,1999)and MEGA 7.0(Kumar et al.,2016).The final alignment included 668 sites.Mean uncorrected genetic distances(P-distances)between sequences were determined with MEGA 7.0.MODELTEST v.3.06(Posada&Crandall,1998)was used to estimate the optimal model of DNA evolution.The best-fitting models selected fortheCOIdataset wereSYM+I for the first,F81+I for the second,and HKY+G for the third codon positions,as suggested by the Akaike Information Criterion(AIC).
Phylogenetic trees were inferred using Bayesian inference(BI)and maximum likelihood(ML).BI was conducted in MrBayes 3.1.2(Huelsenbeck&Ronquist,2001;Ronquist&Huelsenbeck,2003);Metropolis-coupled Markov chain Monte Carlo(MCMCMC)analyses were run with one cold chain and three heated chains for four million generations and sampled every 1 000 generations.Five independent MCMCMC runs were performed and 1 000 trees were discarded as burn-in.We checked the convergence of the runs and that the effective sample sizes(ESS)were all above 200 by exploring the likelihood plots using TRACER v1.5(Rambaut&Drummond,2007).Confidence in tree topology was assessed by posterior probabilities(BPP)(Huelsenbeck&Ronquist,2001).The ML analyses were conducted using Tree finder(Jobb et al.,2004).Confidence in tree topology was tested by non-parametric bootstrap analysis(MLBS)with 1 000 replicates(Felsenstein,1985).Wea prioriregarded tree nodes with bootstrap(MLBS)values of 70%or greater and posterior probabilities(BPP)values over 0.95 as sufficiently resolved,those MLBS between 70%and 50%(BPP between 0.95 and 0.90)as tendencies,and those MLBS below 50%(BPP below 0.90)as unresolved(Felsenstein,2004;Huelsenbeck&Hillis,1993).
RESULTS
Molecular differentiation of Scincella species in Cambodia Sequence data
Final alignment of the examined mtDNACOIgene fragments consisted of 668 sites,with 445 conserved sites and 223 variable sites,of which 220 were parsimony-informative.The transition-transversion bias(R)was 4.59. Nucleotide frequencies were 24.16%(A),29.21%(T),27.82%(C),and 18.81%(G)(data given for ingroup only).
Genealogical relationships and species identification inferred from COI dataset
The BI and ML analyses showed essentially similar topologies(Figure 2),differing only slightly from each other in associations at several poorly supported basal nodes.All six examined species ofScincellaformed six corresponding clades with high levels of node support(BPP=1.0;MLBS=100%).
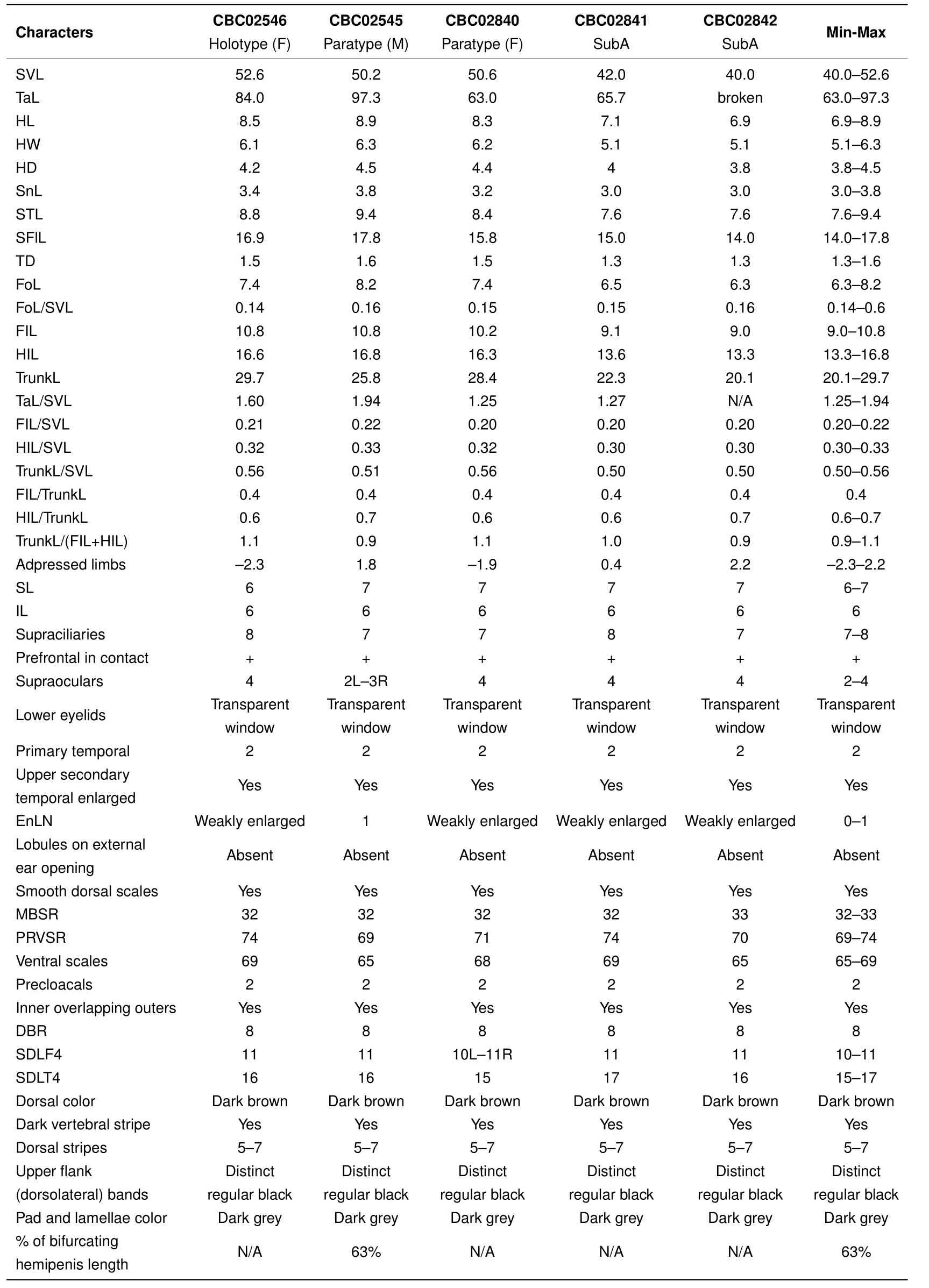
Table 1 Morphometric and meristic characters of Scincella nigrofasciata sp.nov.
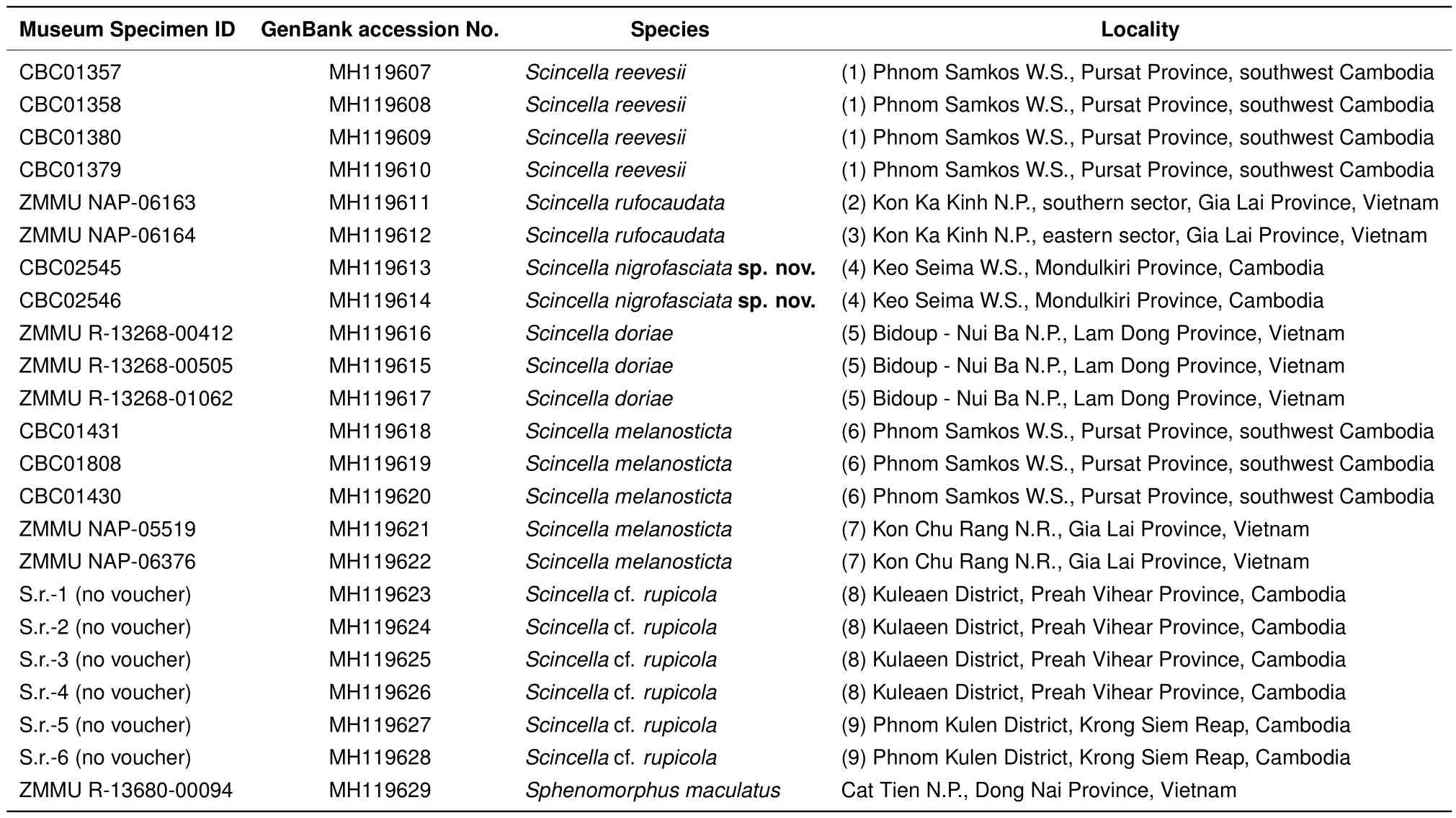
Table 2 Specimens and sequences of Scincella representatives used in molecular analyses of the mtDNA COI gene fragments
The partialCOIgene fragment can be applied as a DNA-barcoding marker,but should not be used as a single tool for reconstructing phylogenetic relationships(Murphy et al.,2013).However,the examined fragment clearly showed thatS.reevesiifrom the Cardamom Mountains in Cambodia,S.rupicolafrom central Cambodia,S.rufocaudatafrom central Vietnam,and the newly discovered population ofScincellafrom Mondulkiri Province formed a well-supported clade(BPP=0.99;MLBS=95%),though phylogenetic relationships within this clade were essentially unresolved.Scincella reevesiifrom Cardamom Mountains andS.rufocaudatafrom central Vietnam were phylogenetically close to each other and represented sister species in our analyses(Figure 2). There was slight differentiation withinS.rupicola,which clustered in two reciprocally monophyletic groups.
Genetic distances
The uncorrected geneticP-distances in the examinedCOIgene fragments among and within the studiedScincellaspecies are shown in Table 3.
The observed interspeci fi c distances in theCOIgene between the examinedScincellaspecies varied fromP=8.84%(betweenS.reevesiiandS.rufocaudata)toP=21.58%(betweenS.rupicolaandS.melanosticta)(Table 3). The observed intraspecific distances in our analysis varied fromP=0.16%toP=2.99%,with the latter value corresponding to genetic differentiation between mtDNA lineages ofS.rufocaudata(Table 3).
Systematics
The newly discovered population ofScincellafrom Mondulkiri Province represents an independent mtDNA lineage,with phylogenetic relationships toS.reevesii,S.rufocaudata,andS.rupicola(Figure 2).This population was clearly distinct inCOIsequences from all examined congeners withP-distances in interspecific comparisons varying from 13.29%(withS.rufocaudata)to 19.92%(withS.melanosticta)(Table 3),indicating deep divergence in the examined mtDNA marker.
Morphologically the Mondulkiripopulation ofScincellaalso showed affinities withS.reevesiiandS.rufocaudata;however,it can be easily diagnosed from these species and other congeners inhabiting the Indochina region by several morphological diagnostic characters(see Comparisons below).Herein,we describe this population as a new species.
Scincella nigrofasciatasp.nov.
Figures 1-8;Tables 1-6.
Holotype:CBC02546,adult female,collected by Thy Neang on 25 September 2016 at N12°19′12.3′′,E107°04′20.8′′,508 m a.s.l in Keo Seima Wildlife Sanctuary,O’Raing District,Mondulkiri Province,Cambodia.
Paratypes:CBC02545,adult male,CBC02840,adult female,and CBC02841-42,two subadults,collected by Thy Neang at the same date and locality as given for the holotype.
Referred materials:CBC02843-45,three juveniles,collected by Thy Neang at the same date and locality as given for the holotype.
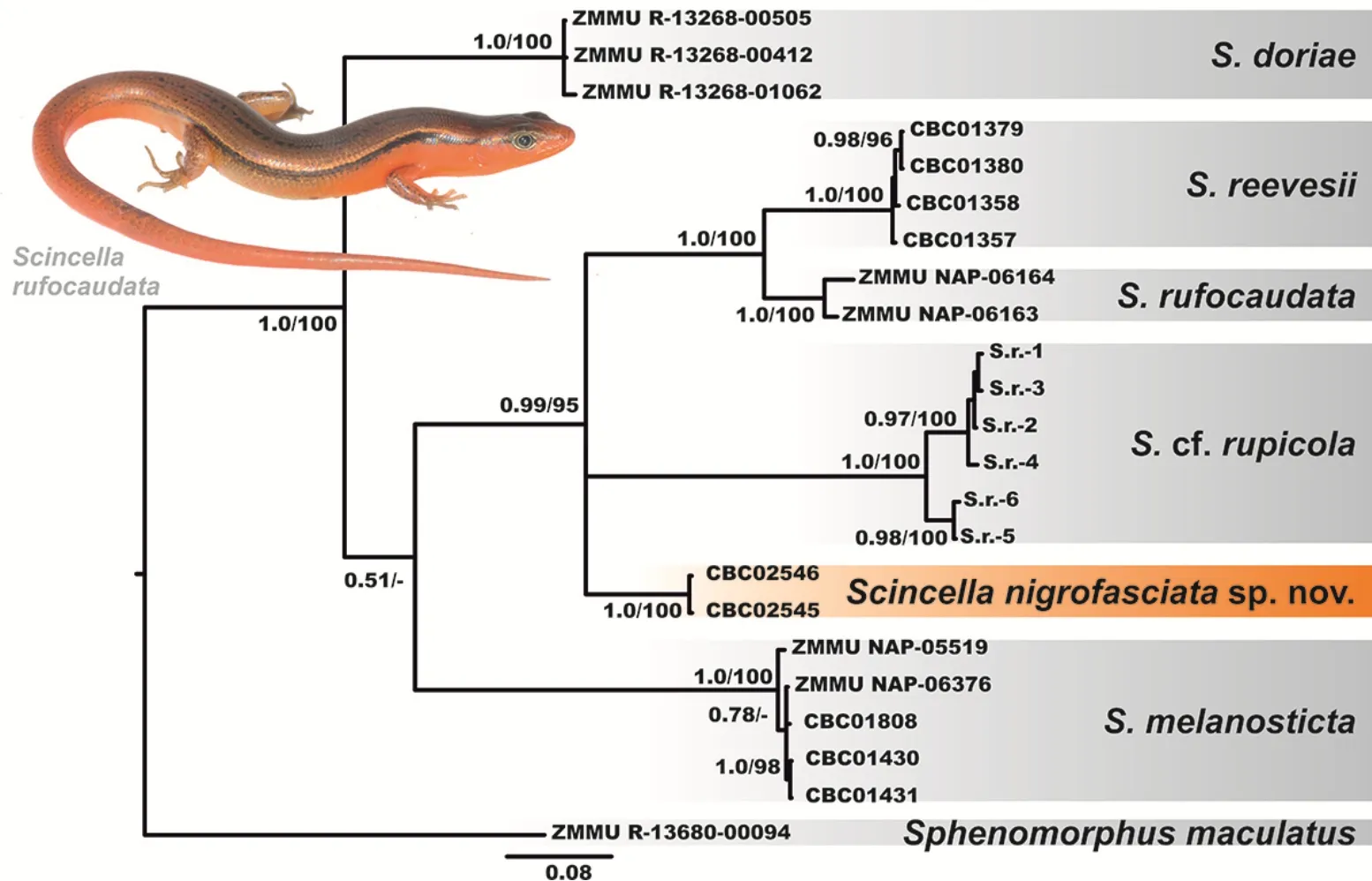
Figure 2 Bayesian inference dendrogram of Scincella derived from analysis of a 668-bp fragment of mtDNA COI gene
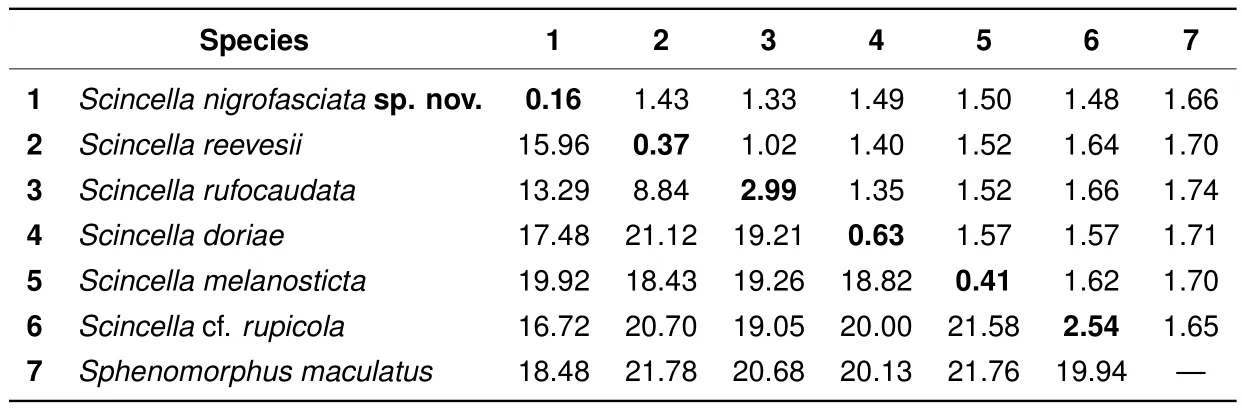
Table 3 Genetic divergence between and within the examined Scincella species
Diagnosis:The new species was assigned to the genusScincellaMittleman,1950 as it shows morphometric and meristic characters matching the diagnosis for this genus.Scincella nigrofasciatasp. nov. can be diagnosed from other congeners by the following combination of morphological attributes:(1)slender and medium-sized,SVL 40.0-52.6 mm;(2)tail relatively long,TaL/SVL(1.25-1.94);(3)FIL/SVL 0.20-0.22;(4)HIL/SVL 0.30-0.33;(5)forelimbs and hind limbs either slightly overlapping(0.4-2.2 mm)or slightly separated(1.9-2.3 mm)when adpressed to body toward each other;(6)infralabials 6;(7)supraciliaries 7-8;(8)prefrontals in broad contact;(9)primary temporals 2;(10)nuchal scales weakly enlarged;(11)external ear opening without lobules;(12)dorsal scales smooth:MBSR 32-33,PRVSR 69-74,VS 65-69,DBR 8;(13)SDLT4 15-17;(14)coloration pattern with dorsum dark brown/greyish-brown in life with 5-7 regular discontinuous dorsal dark stripes(formed by series of dark dots or elongated black spots),including paravertebral stripes,wide black dorsolateral stripes,2-3 scale rows in width,starting from posterior corner of eye and continuing to lateral side of tail,extending 52%-86%of total tail length;and(15)hemipenis bifurcating about 63%of its total length to base.
Description of holotype(Figure 3):A gravid adult female,SVL 52.6 mm;tail relatively long,TaL 84 mm,(TaL/SVL 1.6);head elongated,HL 8.5 mm(HL/SVL 0.16),longer than wide,HW 6.1 mm(HW/HL 0.72),slightly depressed,HD 4.2 mm(HD/HL 0.49).Neck rather slender,slightly distinct from head.
Head:Snout rounded in pro file and dorsal view,SnL 3.4 mm,more than twice as long as TD(1.5 mm);STL 8.8 mm;SFlL 16.9 mm,comprising about one third of SVL;ear vertically oval,TD 1.5 mm;ED 2.5 mm;diameter of cornea 1.3 mm;rostral broad,(width 1.7 mm),almost three times greater than height(0.6 mm),visible from above,in contact with 1stSL laterally,nasals and frontonasal posteriorly;supranasals absent;frontonasal broad,subtrapezoidal in shape,anterior side forming almost straight suture(0.6 mm)with rostral,posterior width 1.7 mm,as wide as rostral,little more than twice as wide as length(0.8 mm),in contact with nasals and 1stloreal laterally,posterior margin slightly overlapping prefrontals;prefrontals in broad contact,laterally bordered by two loreals,frontal posteriorly;frontal elongated(length 2.8 mm),kite-shaped,posterior part much longer than anterior;greatest width anteriorly 1.4 mm,twice as narrow as length(width/length 0.5);frontal in contact with 1stand 2ndsupraoculars laterally,frontoparietals posteriorly,anterior corner of rostral end slightly separating posterior portions of prefrontals medially,posterior corner of frontal slightly overlapping medial suture between frontoparietals;frontoparietals two,each diamond-shaped,together forming a butter fl y-shape with median suture 1.2 mm,in contact with 2nd,3rd,and 4thsupraoculars laterally,interparietal and parietals posteriorly;interparietal rather small,kite-shaped,with posterior portion little longer than anterior,in contact with parietals posteriorly,anterior corner of interparietal acute,slightly intruding into median suture between frontoparietals;parietals large,in contact with each other posteriorly(suture 0.6 mm behind posterior corner of interparietal),narrowly contacting 4thsupraocular and posterior supraciliary scale,in broad contact with upper secondary temporal laterally and four nuchal scales posteriorly.Naris rounded,laterally pierced in nasal scale;nasals in contact with 1stSL ventrally,frontonasal dorsally,1stloreal posteriorly;loreals two,anterior loreal rhomboidal,in contact with 2ndSL ventrally,frontonasal and prefrontal dorsally,posterior loreal subtrapezoidal,in contact with 2ndand 3rdSL ventrally,preocular and upper presubocular posteriorly,prefrontal and anterior supraciliary scale dorsally;preocular one,elongate,triangular,in contact with anterior supraciliary scale dorsally,anterior edge of orbit posteriorly,anterior presubocular ventrally;supraciliaries eight,anterior two largest;supraoculars four, fi rst two contacting frontal,second to third contacting frontoparietal;presuboculars three,posterior-most slightly intruding into suture between 3rdand 4thSL,anterior-most triangular,slightly larger than posterior-most presubocular; suboculars two,both contacting 4thSL,anterior slightly overlapping posterior one,slightly overlapped by lower presubocular,posterior broadly overlapping lower postsubocular,both bordered above by granular scales of lower eyelid;postoculars two;postsuboculars four(left side)and fi ve(right side),lowest slightly intruding between 4thand 5thSL,uppermost largest;lower eyelid with distinct transparent disc(window)bordered above by small palpebral scales;supralabials six,1stsmallest,4thlocated ventral to window of eye,5thlargest;infralabials six,1stsmallest,5thlargest;primary temporals two,lower larger,sub-rhomboid,anteriorly in contact with 3rdand 4thpostsuboculars,ventrally with 5thand 6thSL,posteriorly with lower secondary temporal,upper primary temporal subrhomboid,anteriorly in contact with 1stand 2ndpostsubocular anteriorly,posteriorly with both secondary temporals;secondary temporals two,lower smaller,overlapping upper,in contact with 6thSL ventrally,upper secondary temporal about twice as large as lower,in contact with posterior-most postsubocular anteriorly,with parietal dorsally and nuchal scale posteriorly;nuchal scales four,bordering posterior edge of parietals,slightly enlarged in comparison with adjacent posterior scales.Mental rounded,width(1.6 mm)more than twice as wide than long(0.7 mm),in contact with 1stIL laterally,postmental posteriorly;postmental large,width(2.0 mm)greater than length(1.1 mm),in contact with 1stand 2ndIL laterally,1stchinshield posteriorly;chinshields in three pairs,1stpair in broad contact median with each other,contacting 3rdIL laterally,2ndpair separated by subtriangular gular scale,contacting 4thIL laterally,3rdpair separated medially by three gular scales,in contact with 5thand 6thIL laterally and three gular scales posteriorly.
Body,limbs,and tail:Body scales smooth,cycloid,imbricate;dorsal scales between dorsal stripes?+8+?,same size as ventral scales,slightly larger than those on body sides and gular scales;scales on anterior fl anks between tympanic region and posterior margin of axilla smaller than adjacent dorsal scales;MBSR 32;PRVSR 74;VS 69;enlarged preanal scales two,median scales overlapping outer;subcaudal scales 111,anterior in three rows,reducing to two at quarter of tail length and one row about half way to tail tip,slightly larger than surrounding scales.Trunk relatively long,TrunkL 29.7 mm,little more than half of SVL,more than addition of FIL and HIL,ratio of TrunkL/(FIL+HIL)1.1;forelimb short,FIL 10.8 mm(FIL/SVL 0.21);forearm short,rather slender,FoL 7.4 mm(FoL/SVL 0.14);hindlimb longer than forelimb,HIL 16.6 mm(HIL/SVL 0.32),limbs separated by 2.3 mm when adpressed(4.4%of SVL);digits slender;SDLF4 11;SDLT4 16.
Coloration in life:In life,the female holotype CBC02546 had the same color as female paratype CBC02840(Figure 4). Dorsal surface of head,dorsum,and base of tail dark bronze-brown,side of head between tip of snout and forelimb insertion dark brown;dorsal surface of remaining tail reddish-brown.Dark broken regular dorsal stripes anteriorly and on tail(5)and posteriorly on body(7),formed by series of dark dots or elongated black spots,including wider dark paravertebral stripe;anterior part of dorsum with dorsal stripes formed by series of dots,posterior part of dorsum with dark dot-formed regular dorsal stripes reaching base of tail,continuing with dark stripes on dorsal surface of tail,extending about one-third of tail length;light laterodorsal stripes from behind eye,through temporals,along dorsolateral scale row to lateral sides of tail,and fading at one-third of tail length;large distinct regular black longitudinal dorsolateral stripe on each side of body,covering two to three scale rows,starting as narrow stripe covering about one scale row,running from posterior corner of eye through upper temporals,above tympanum,expanding wider to two scale rows above axilla,running below light dorsolateral stripe,along upper fl anks through upper angle of groin to lateral surface of tail,becoming indistinct at posterior lateral tail(~12 mm from tail tip);body lf anks ventrally with whitish-beige longitudinal streaks and dark markings on bluish-brown background;ventrolateral surfaces from below level of eye to axillary region with longitudinal whitish-grey streaks and dark marking on reddish-brown background;lateral surfaces of tail reddish-brown;dorsal surfaces of limbs with irregular dark blotches on dark brown background. Ventral surfaces of head,gular region,body,and limbs uniformly white;ventral surface of tail uniform pinkish-cream.Palmar surfaces of hands and thenar surfaces of feet dark grey.Iris light-grey.
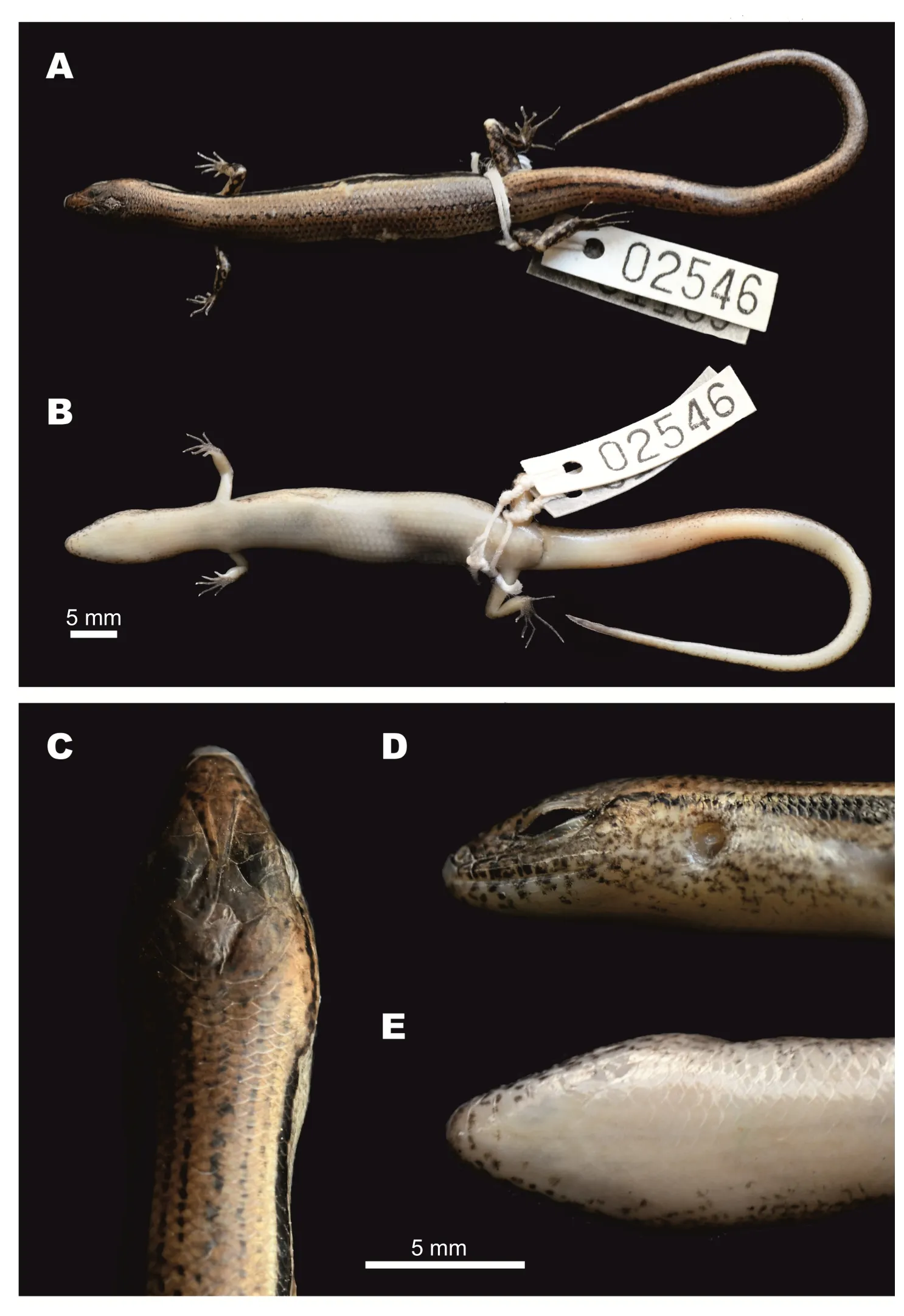
Figure 3 Female holotype of Scincella nigrofasciata sp. nov. (CBC02546) in preservative
Coloration in preservative:In preservative,dorsal surfaces of holotype turned dark greyish-brown;wide dorsolateral black stripe remained distinct;head with faint irregular dark spots,pineal ocellus present as single white dot on posterior part of interparietal;sides of head and supralabials with dark mottling;infralabials with dark spots;lateral sides of tail with small dark spots;throat and ventral surface of body and limbs greyish-cream;ventral surface of tail lighter cream;palmar surface of hand, fingers,and toes dark grey(Figure 3,Figure 7D,E,F).
Variation:Paratypes (Table 1)resemble holotype in most morphometric and meristic characters and coloration.Noteworthy variation is that the holotype has six SL on each side of the head,whereas all paratypes have seven.CBC02545 has two supraoculars on left side,second posterior one larger with clear short suture indicating incomplete fusion of two posterior supraoculars;three supraoculars on right side,third with clear short suture indicating incomplete fusion with fourth posterior-most supraocular;one distinct pair of enlarged nuchal scales(remaining specimens have weakly enlarged nuchals)and first pair of chinshields in narrow contact with each other(vs.in broad contact in other specimens).CBC02545 has four postsuboculars and CBC02840 has five postsuboculars on both sides.Male paratype exhibits greater tail length(97.3 mm)than female holotype and paratypes.All specimens have six nuchal scales posterior to parietals,except subadult CBC02842,which has seven.In life,male shows distinctly more reddish-brown coloration of dorsum,with indistinct mid-dorsal stripe;throat,lower body flanks,ventral surfaces of body,lateral and ventral surfaces of tail in male show distinct reddish-orange coloration(Figure 4B).In preservative,reddish-orange coloration faded and became paler(Figure 7D).
Natural history:The species was recorded from semi-deciduous lowland forests at elevations ca.400-500 m a.s.l.in Keo Seima Wildlife Sanctuary from the eastern plain of Cambodia.Most specimens were encountered during the day,but juveniles CBC02843-45 were encountered at night among leaf litter.The holotype female CBC02546,paratype male CBC02545,and subadult CBC02841 were spotted moving near rotten logs,female CBC02840 was moving along the ground on the forest floor,and subadult CBC02842 was found under a rotten log.Diet and reproductive biology of the new species remain unknown.The gravid female holotype carried two eggs.
Etymology:The specific epithet is from the Latin words “niger”for“black”and “fascia”for“band”,in reference to the wide black dorsolateral stripes typical for this species.
Distribution:To date known only from the type locality in Keo Seima Wildlife Sanctuary,O’Raing District,Mondulkiri Province,Cambodia at elevations ca.400-500 m a.s.l..However,the discovery of this species in adjacent areas of southern Vietnam is highly expected.
Comparisons:The morphological characters distinguishing the new species from its Southeast Asian congeners are summarized in Table 4. Morphological comparisons ofScincellaspecies found in Cambodia are given in Table 5.Scincella nigrofasciatasp.nov.can be diagnosed fromS.apraefrontalisNguyen,Nguyen,B?hme and Ziegler,2010 of Vietnam by its longer SVL(40.0-52.6 vs.36.1 mm),greater number of IL(6 vs.5),DBR(8 vs.4),MBSR(32-33 vs.18),PRVSR(69-74 vs.52),and VS(65-69 vs.50),and prefrontals in broad contact(vs.prefrontals absent);fromS.monticola(Schmidt,1925)of Vietnam and China by having a longer SVL(40.0-52.6 vs.31.8 mm),two primary temporals(vs.one),fewer EnLN(0-1 vs.3-4),and greater number of DBR(8 vs.4),MBSR(32 vs.22-26),PRVSR(69-74 vs.52-59),VS(65-69 vs.52-58),and SDLT4(15-17 vs.10-13);and fromS.punctatolineata(Boulenger,1893)of Thailand and Myanmar by longer SVL(40.0-52.6 mm for three adults and single subadult specimen,SVL 50.2-52.6 mm for three adults vs.37.6-40.2 mm),greater number of MBSR(32-33 vs.22-28),two primary temporals(vs.one),and greater number SDLT4(15-17 vs.13-15).Scincella nigrofasciatasp.nov.can be distinguished fromS.darevskiiNguyen,Ananjeva,Orlov,Rybaltovsky and B?hme,2010 of Vietnam by having a much shorter SVL(40.0-52.6 vs.88.6 mm),fewer supraoculars(2-4 vs.5),two primary temporals(vs.one),and greater number of DBR(8 vs.6),MBSR(32-33 vs.28),and PRVSR(69-74 vs.62);fromS.doriae(Boulenger,1887a)of Myanmar and China by having a shorter SVL(40.0-52.6 vs.58.6 mm),fewer EnLN(0-1 vs.3-5),slightly fewer VS(65-69 vs.70-79),5-7 discontinuous regular dark dorsal stripes(vs. dorsum caramel brown with small brown spots),and distinct wide black dorsolateral stripes(vs.dark brown dorsolateral stripes broken up by whitish spots);fromS.raraDarevsky&Orlov,1997 of central Vietnam by having fewer EnLN(0-1 vs.3),greater number of MBSR(32-33 vs.24)and PRVSR(69-74 vs.53),and single row of basal subdigital pads(vs.double row of basal subdigital pads);fromS.victoriana(Shreve,1940)of Myanmar by having a shorter SVL(40.0-52.6 vs.57.5 mm),fewer EnLN(0-1 vs.3),smooth dorsal scales(vs.keeled),and a greater number of PRVSR(69-74 vs.50-54)and VS(65-69 vs.53-56).The new species can be distinguished fromS.ochracea(Bourret,1937)of Vietnam and Laos by its longer SVL in males(50.2 mm,n=1 vs.34.2-45.4 mm,n=6),lack of lobules around external ear opening(vs.2-4 lobules),dark brown dorsum with 5-7 discontinuous regular dark dorsal stripes(vs.silver-grey with a dark vertebral stripe),and distinct wide black dorsolateral stripes(vs.dark brown flanks broken up by light spots).
Among the Cambodian species,Scincella nigrofasciatasp.nov.can be distinguished fromS.melanosticta(Boulenger,1887b)of Cambodia,Myanmar,Thailand,and Vietnam by its comparatively shorter forelimbs(FIL/SVL 0.20-0.22 vs. 0.23-0.27),comparatively shorter hind limbs(HIL/SVL 0.30-0.33 vs. 0.35-0.37),adpressed limbs overlapping 0.4-2.2 mm in males and subadult specimens and separated by a 1.9-2.3 mm gap in adult females(vs.adpressed limbs widely overlapping 4.5-8.2 mm),two primary temporals(vs.one),fewer DBR(8 vs.10),and 5-7 discontinuous regular dark dorsal stripes(vs.dark brown dorsum with dark dense spots without obvious striped pattern).The new species can be distinguished fromS.rupicolaby its comparatively shorter hind limbs(HIL/SVL 0.30-0.33 vs. 0.36-0.40),adpressed limbs overlapping 0.4-2.2 mm in male and subadult specimens and separated by a 1.9-2.3 mm gap in adult females(vs.adpressed limbs overlapping 3.5-7.2 mm),fewer SDLT4(15-17 vs.18-21),fully everted hemipenis bifurcating at 63%of total hemipenis length,n=1,Figure 5E(vs. 69%-77%,n=3,Figure 5F),comparatively more slender fingers and toes(Figure 5A vs.Figure 5B),5-7 discontinuous regular dark dorsal stripes,Figure 4,Figure 7D-F(vs.dark blotches on dorsum in females and uniform reddish brown pattern without dark markings in males inS.rupicola;Figure 6A-B,Figure 7B-C).
In both morphometric and meristic charactersScincella nigrofasciatasp. nov. is most similar toS.reevesiiandS.rufocaudata. However,the new species can be distinguished fromS.reevesiiby slightly shorter forelimbs(FIL/SVL 0.20-0.22 vs. 0.24-0.30),generally shorter hind limbs(HIL/SVL 0.30-0.33 vs. 0.34-0.43),comparatively shorter forearms(FoL/SVL 0.14-0.16 vs.0.17-0.19,Table 5),adpressed limbs overlapping 0.4-2.2 mm in males and subadult specimens and separated by a distance of 1.9-2.3 mm in females(vs.overlapping 3.9-6.5 mm in both sexes),5-7 dark discontinuous regular dark dorsal stripes(vs.irregular dark vertebral line and dark dorsal spots),wide distinct black dorsolateral stripes,continuing to lateral sides of tail,Figure 4(vs.dark dorsolateral stripes less distinct and broken up by light spots and only extending to tail base in both sexes,Figure 6E,D),comparatively more slender fingers and toes(Figure 5A vs.Figure 5C),and dark brown palmar surfaces of hands and lower surface of fingers and toes,Figure 5A(vs.light grey palmar surfaces of hands, fingers and toes,Figure 5C).
Scincella nigrofasciatasp. nov. can be distinguished fromS.rufocaudataby prefrontals in broad contact(vs.prefrontalsseparated),comparatively shorterhind limbs,(HIL/SVL 0.30-0.33 vs.0.37),fewer IL(6 vs.7),fewer DBR(8 vs.10,Tables 4,5,6),5-7 discontinuous regular dark dorsal stripes(vs.1-3 dark stripes with spots,Figure 8),and distinct wide black dorsolateral stripes continuing along tail(vs.black stripes broken,ending at tail base,Figure 4)(Table 5).
DISCUSSION
Ourworkclearlydemonstratedthe new speciesfrom Mondulkiri Province to be distinct from otherScincellaspecies known from Cambodia,includingS.melanosticta,S.reevesii,S.cf.rufocaudata,and S.rupicola.Data on their morphological differences are summarized in Tables 4-5 and Figures 5-7.As the original description ofSphenomorphus rufocaudatusby Darevsky&Nguyen(1983)was quite short and published only in Russian,we provided additional morphological information(Tables 5 and 6)and photos(Figure 8)of the holotype ZISP 19797.
To facilitate future work on the genusScincellaof Cambodia,we provide the following comparisons between the named species,based on specimen examination and character states taken from the literature(Table 4).Scincella melanostictacan be distinguished fromS.reevesiiby one primary temporal(vs. two)and greater number of DBR(10 vs. 8). Both male and femaleS.melanostictahave a dorsum with dense dark spots(dark spots on almost every dorsal scale)and lack conspicuous dorsal stripes(vs.vertebral/irregular lines of dorsal spots inS.reevesii,except some male individuals ofS.reevesii,probably in the breeding season,show a reddish brown dorsum that lacks dorsal spots),and distinct dark dorsolateral stripes interrupted by irregular light transverse bars/spots(vs.dark dorsolateral stripes,running along upper flanks and less interrupted by light spots).The bifurcated part of the hemipenis is 56%its total length(n=1)inS.melanosticta(vs.65%-68%,n=2,inS.reevesii).
Scincellamelanostictacan be distinguished fromS.rufocaudataby prefrontals in contact(vs. prefrontals separated),one primary temporal(vs.two),longer forearms(FoL/SVL ratio 0.17-0.20 vs.0.15),SDLT4 21-22(vs.15),dorsal dark brown with dense pattern of dark spots(vs.ochre with 1-3 dark stripes and spots),and dark dorsolateral stripes interrupted by irregular light transverse bars/spots in both sexes(vs.distinct,regular dark dorsolateral stripes(Figures 6-7 forS.melanosticta).
Scincella melanostictacan be differentiated fromS.rupicolaby having one primary temporal(vs.two),greater number of DBR(10 vs.8),dorsum with dense dark dorsal spots in both sexes(vs.dark blotches with pair of smaller blotches on neck in females only,whereas males lack blotches or have very faint dark dorsolateral stripes),and heavy dark spots on sides of head,extending above axillary region and below dorsolateral stripes along flanks in both sexes inS.melanosticta(vs.dark mottling in females and no dark markings in males inS.rupicola(Figures 6A-C;7A-C).
Scincella reevesiican be distinguished fromS.rupicolaby its higher TaL/SVL ratio 1.57-1.73,n=3(vs.1.06-1.53,n=5),dark brown dorsum with small vertebral/irregular dorsal spots(vs.dorsal blotches and pair of smaller blotches on neck only in females),distinct wide dark dorsolateral stripes,running along upper flanks,almost not interrupted by light spots(vs.large dark blotches interrupted by light bars in females),and body slender in males(vs.body in males comparatively thicker inS.rupicola)(Figure 6A,B,D,E;Figure 7G-H).
Scincella rufocaudatacan be distinguished fromS.rupicolaby prefrontals separated(vs. in broad contact),shorter forelimbs(FlL/SVL 0.22 vs. 0.23-0.26),adpressed limbs overlapping at about 1.8 mm(vs.3.5-7.2 mm),dorsal ochre with 1-3 dark brown spots(vs.dark brown dorsal pattern with blotches in females and without blotches in males),and slender body(vs.comparatively thicker inS.rupicola).The dorsal pattern with dark blotches and a few pairs of smaller blotches on neck in females and characters stated in Table 4 match the diagnosis ofS.rupicolaby Taylor(1963).Scincella rupicolahas been reported from Thailand,Laos,and Vietnam(Nguyen et al.,2010b;Taylor,1963;Teynié et al.,2004),but has not been reported previously from Cambodia.Herein we identify this species asS.cf.rupicolafor the first time from Cambodia.This species ranges from the central part,Kampong Thom(Hayes et al.,2015)to Siem Reap Province(T.Hartmann,pers.comm.).

Figure 4 Coloration in life of Scincella nigrofasciata sp. nov. (Photos by Thy Neang)
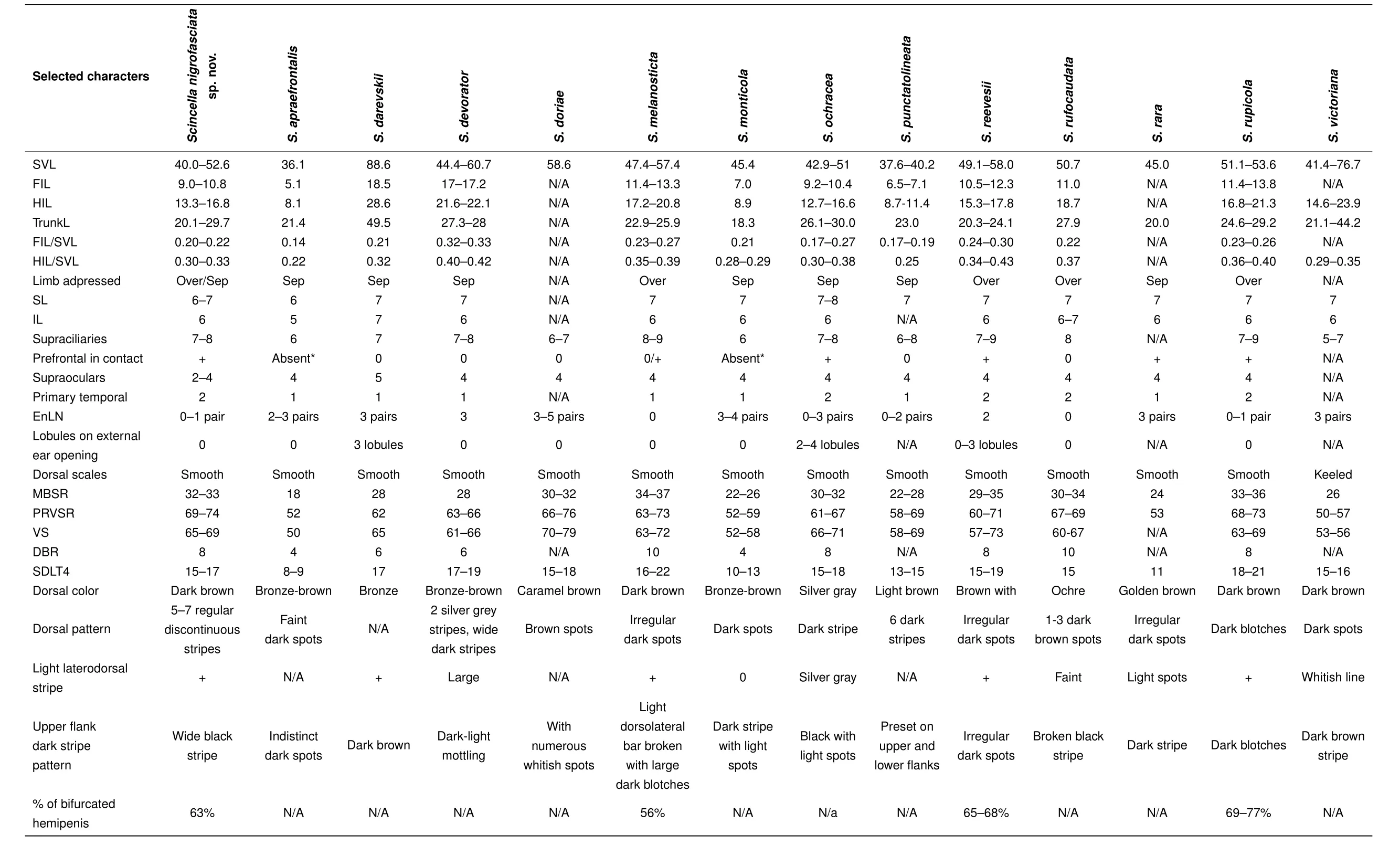
Table 4 Comparison of diagnostic morphometric and meristic characters of the new species and other Scincella species of Southeast Asia
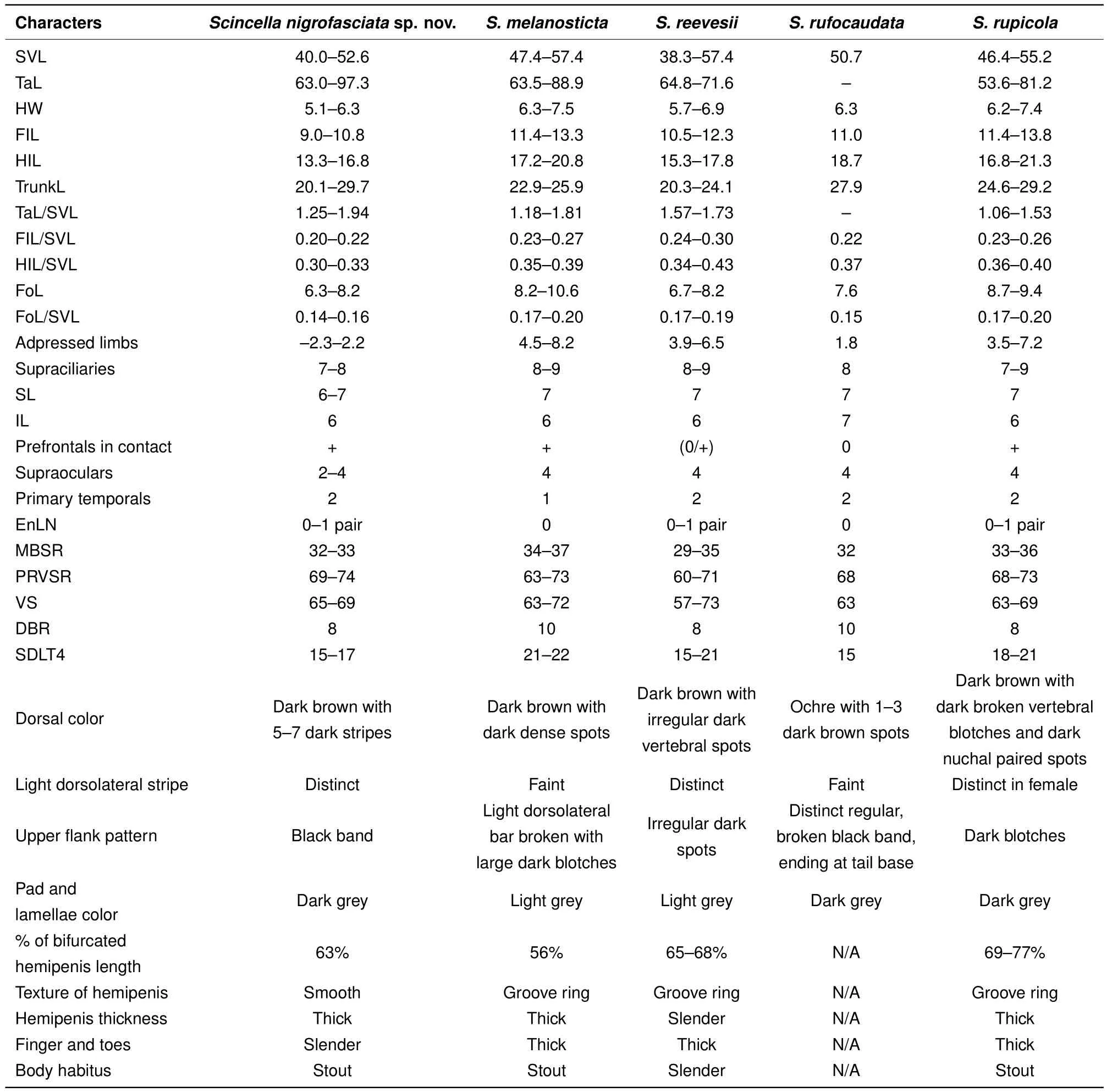
Table 5 Morphometric and meristic character comparisons between Scincella species from Cambodia and Scincella rufocaudata from Vietnam
Morphologically,S.reevesiiis super fi cially similar toS.rufocaudata(Tables 4-5). It can be distinguished fromS.rufocaudataby its prefrontals in broad contact(vs.separated),adpressed limbs more widely overlapping(3.9-6.5 vs.1.8 mm),upper secondary temporal enlarged(vs.not enlarged),and DBR 8(vs.10);see Tables 5-6 for more detail.
We examined photographs of specimens(FMNH 263355-58)from the Cardamom Mountains of southwest Cambodia,deposited at the Field Museum of Natural History,which were assigned toS.rufocaudataby Stuart&Emmett(2006),and suggest that these specimens appear more likeS.reevesiithanS.rufocaudatabased on their dark dorsolateral stripes,which are more continuous and interrupted by less distinct light spots/bars. Two(CBC01380 and CBC02305)out of seven male specimens from the Cardamom Mountains of southwest Cambodia that we assigned toS.reevesiihave prefrontals slightly separated and another male(CBC01379)has prefrontals in narrow contact,which we suggest is a variation within this population ofS.reevesii.
We examined photographs of specimens (FMNH 262998-99)collected 3-4 km from the new species type locality in Mondulkiri on the Cambodian eastern plain,which were assigned toS.rufocaudataby Stuart et al.(2006).These specimens look different fromS.rufocaudata,based on their prefrontals in contact as opposed to prefrontals separated inS.rufocaudata;and differ fromS.reevesiiin having more distinct wide dark dorsolateral stripes;they also differ from the new species based on their light brown dorsal coloration,and lower edge of dorsolateral stripes more interrupted by irregular light spots.However,because these specimens were inaccessible to us,we suggest retaining the specimens FMNH262997-3001 reported by Stuart et al.(2006)from Mondulkiri asScincellacf.rufocaudata,pending further studies and genetic analyses.
The discovery of a new species ofScincellain Mondulkiri Province brings the named species ofScincellaknown for Cambodia to fi ve,namely,Scincella nigrofasciatasp.nov.,S.melanosticta,S.reevesii,S.cf.rufocaudata,andS.cf.rupicola,adding another species of lizard to the country. The new species seems to be morphologically quite variable;therefore,additional adult specimens and further studies are required to assess morphometric and meristic character variations.
To date,Scincella nigrofasciatasp.nov.is known only from the type locality in the Mondulkiri Province of Cambodia.However,it is very likely that the new species inhabits other hilly areas of the southern outcrops of the Annamite Mountains in adjacent areas of Vietnam(e.g.,Binh Phuoc,Lam Dong,and Dong Nai Provinces),and thus further morphological and molecular studies are needed to con firm the extent of its distribution in southern Indochina.The discovery of a new species ofScincellaindicates that the reptile fauna of Keo Seima Wildlife Sanctuary remains insufficiently studied and future field surveys are needed to assess its herpetodiversity.This study further highlights the importance of taxonomic research and biodiversity assessments for nature conservation.
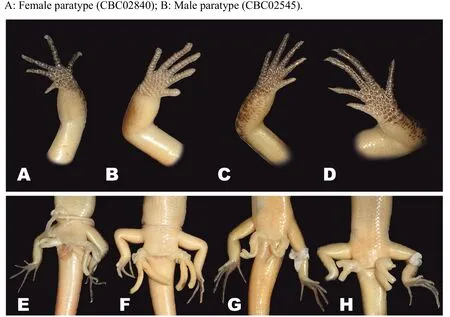
Figure 5 Morphology and coloration of fingers(A-D)and structure of hemipenes(E-H)of Cambodian Scincella species(Photos by Thy Neang)

Figure 6 Differences in dorsal and dorsolateral views in life between Scincella species from Cambodia(Photos by Thy Neang)
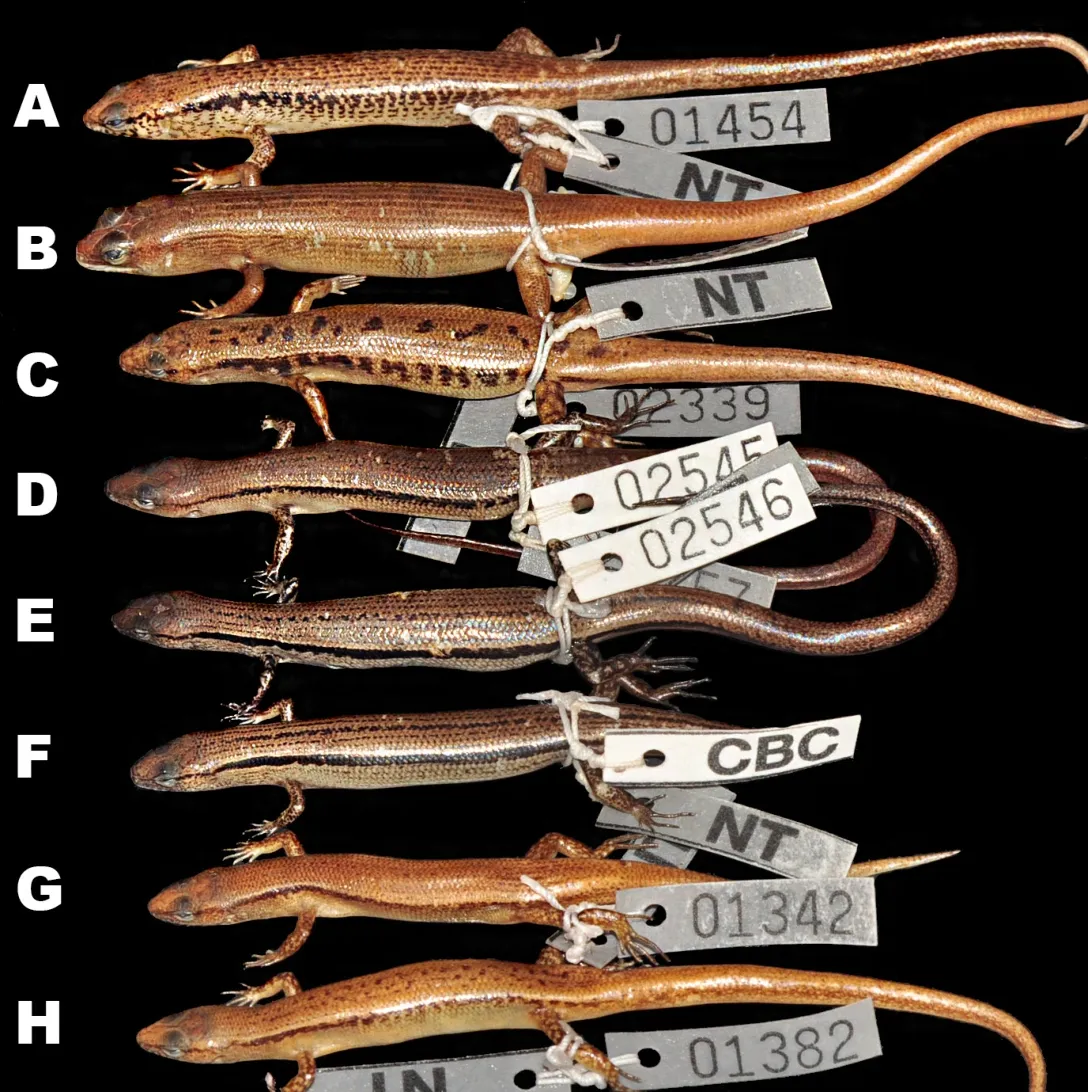
Figure 7 Dorsolateral views of representatives of Cambodian Scincella species(in preservative)(Photos by Thy Neang)
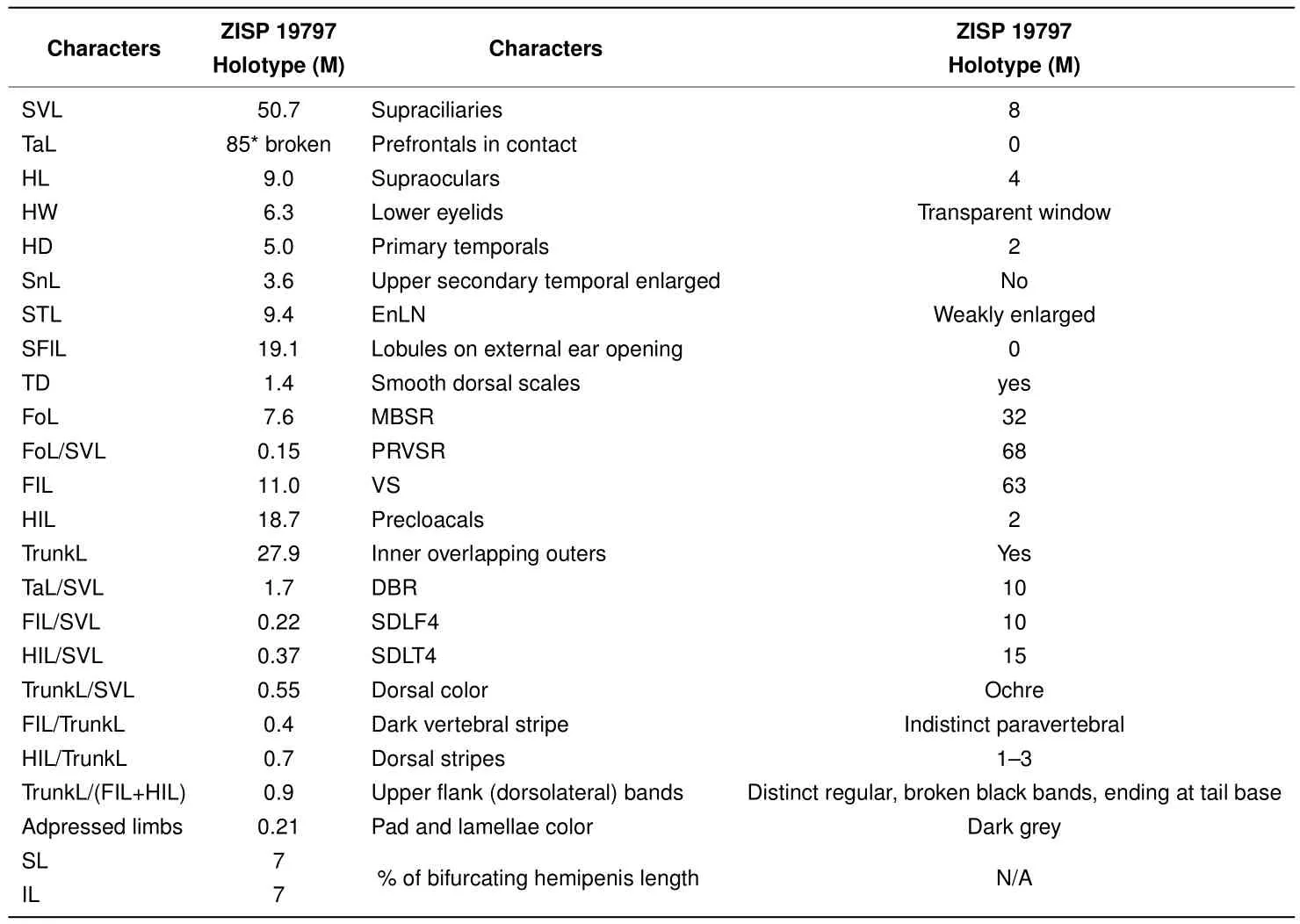
Table 6 Morphometric and meristic characters of the holotype of Sphenomorphus rufocaudatus Darevsky&Nguyen,1983(ZISP 19797;now Scincella rufocaudata)
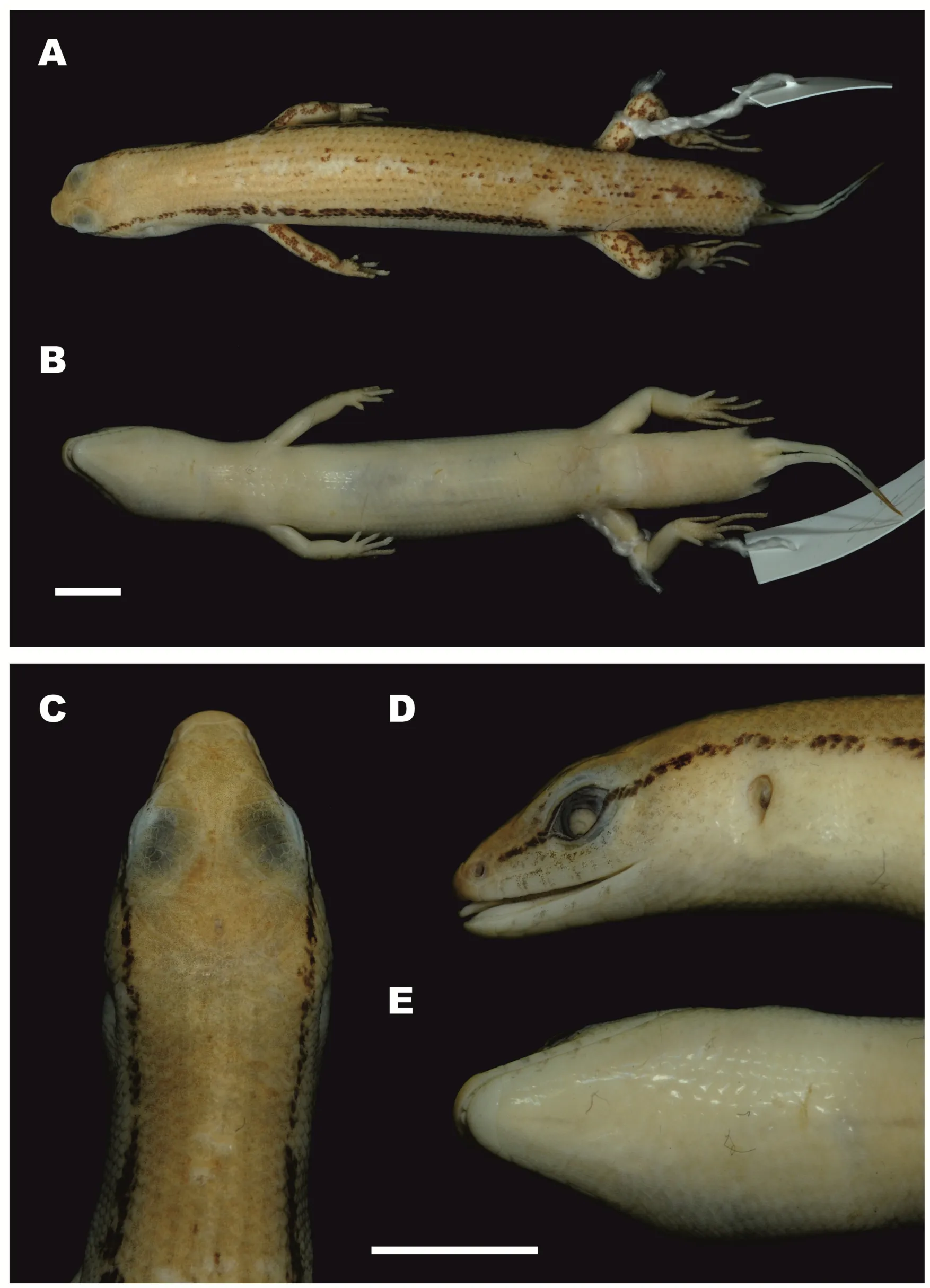
Figure 8 Male holotype of Sphenomorphus rufocaudatus(Darevsky&Nguyen,1983)(ZISP 19797;now Scincella rufocaudata)(Photos by Nikolay A.Poyarkov)
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’CONTRIBUTIONS
T.N.and N.A.P.designed the study.T.N.and S.C.collected data.N.A.P.performed molecular experiments. T.N.examined morphology. N.A.P.conducted phylogenetic analyses.T.N.and N.A.P.wrote the manuscript,T.N.and S.C.revised the manuscript.All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank H.E Say Sam Al and H.E Chea Sam-Ang for p ermission to conduct the field survey and for support in research and conservation.Thanks are also given to Tuy Sereivathana and Ith Saveng for facilitation and support for museum and laboratory work.We thank Kung Putheara and Nuth Menghor for field work facilitation.We also thank Nguyen Quang Truong,Bryan Stuart,and Gernot Vogel for providing literature.We are sincerely grateful to Timo Hartmann and Peter Geissler for providing useful comments and information.We are sincerely grateful to Glenn Shea for photographs and useful comments on the earlier version of this manuscript.We are deeply indebted to Olivier S.G.Pauwels(Institut Royal des Sciences Naturelles de Belgique,Brussels),Patrick David(Musée National d’Histoire Naturelle,Paris),and an anonymous reviewer for revisions,which helped to improve this manuscript.We are grateful to Natalia B.Ananjeva and Nikolai L.Orlov(ZISP,St.Petersburg,Russia)and Valentina F.Orlova(ZMMU,Moscow,Russia)for permission to examine specimens under their care and to Konstantin D.Milto(ZISP,St.Petersburg,Russia)and Duong Van Tang(MSU,Moscow,Russia)for assistance.
REFERENCES
Amarasinghe AAT,Poyarkov NA,Campbell PD,Leo S,Supriatna J,Hallermann J.2017.Systematics ofEutropis rugifera(Stoliczka,1870)(Squamata:Scincidae)including the redescription of the holotype.Zootaxa,4272(1):103-118.
Boulenger GA.1887a.An account of the Scincoid lizards collected from Burma for the Genoa Civic Museum by Messrs.G.B.Comotto and L.Fea.Annali del Museo Civico di Storia Naturale di Genova.Serie 2,4:618-624.Boulenger GA.1887b.An account of the reptiles and batrachians obtained in Tenasserim by M.L.Fea of the Genoa Civic Museum.Annali del Museo Civico di Storia Naturale di Genova.Serie 2,5:474-486.
Boulenger GA.1893.Viaggio di Leonardo Fea in Birmania e Regioni Vicine LII.Concluding report on the reptiles and batrachians obtained in Burma by Signor L.Fea,dealing with the collection made in Pegu and Karin Hills in 1887-88.Annali del Museo Civico di Storia Naturale di Genova.Serie 2,13:304-347.
Bourret R.1937.Notes herpétologiques sur l’Indochine fran?aise.XII Les lézards de la collection du Laboratoire des Sciences Naturelles de l’Université.Bulletin général de l’Instruction Publique,Hanoi,1937:1-39.
Burland TG.1999.DNASTAR’s lasergene sequence analysis software.Methods in Molecular Biology,132:71-91.
Darevsky IS,Nguyen VS.1983.New and little known lizard species from Vietnam.Zoologichesky Zhurnal,62:1827-1837.(in Russian)
Darevsky IS.1990.Notes on the reptiles(Squamata)of some off shore islands along the coast of Vietnam.In:Peters G,Hutterer R.Vertebrates in the Tropics:Proceedings of the Symposium on Vertebrate Biogeography and Systematics in the Tropics.Bonn,Germany:Museum Alexander Koenig,125-129.
Darevsky IS,Orlov NL.1997.A new genus and species of scincid lizards from Vietnam:the first Asiatic skink with double rows of basal subdigital pads.Journal of Herpetology,31(3):323-326.
Darevsky IS,Orlov NL,Ho TC.2004.Two new lygosomine skinks of the genusSphenomorphusFitzinger,1843(Sauria,Scincidae)from northern Vietnam.Russian Journal of Herpetology,11(2):111-120.
Felsenstein J.1985.Confidence limits on phylogenies:an approach using the bootstrap.Evolution,39(4):783-791.
Felsenstein J. 2004. Inferring Phylogenies. 2nded. Sunderland,Massachusetts:Sinauer Associates,Inc.Publishers,465.
García-Vázquez UO,Canseco-Márquez L,De Oca ANM.2010.A new species ofScincella(Squamata:Scincidae)from the Cuatro Ciénegas Basin,Coahuila,Mexico.Copeia,2010(3):373-381.
Gonzalez M,Lwin KS,Vindum JV.2005.New records forScincella Victoriana(Shreve,1940)from the Chin Hills,Myanmar.Proceedings of the California Academy of Sciences,56(26):391-392.
Greer AE.1974.The genetic relationships of the scincid lizard genusLeiolopismaand its relatives.Australian Journal of Zoology Supplementary Series,22(31):1-67.
Greer AE,Shea G.2003.Secondary temporal scale overlap pattern:a character of possible broad systematics importance in Sphenomorphine skinks.Journal of Herpetology,37(3):545-549.
Grismer LL,Chav T,Neang T,Wood Jr PL,Grismer JL,Youmans TM,Ponce A,Daltry JC,Kaiser H.2007.The herpetofauna of the Phnom Aural Wildlife Sanctuary and checklist of the herpetofauna of the Cardamom Mountains,Cambodia.Hamadryad,31(2):216-241.
Grismer LL,Neang T,Chav T,Grismer JL.2008.Checklist of the amphibians and reptiles of the Cardamom region of southwestern Cambodia.Cambodian Journal of Natural History,2008(1):12-28.
Hall TA.1999.BioEdit:a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT.Nucleic Acids Symposium Series,41(2):95-98.
Hartmann T,Geissler P,Poyarkov Jr NA,Ihlow F,Galoyan EA,R?dder D,B?hme W.2013.A new species of the genusCalotesCuvier,1817(Squamata:Agamidae)from southern Vietnam.Zootaxa,3599(3):246-260.Hayes B,Khou EH,Neang T,Furey N,Sophea C,Holden J,Seiha H,Sarith S,La P,Simpson V.2015.Biodiversity Assessment of Prey Lang Kratie,Kampong Thom,Stung Treng and Preah Vihear provinces.Unpublished Report to CI.
Hillis DM,Moritz C,Mable BK.1996.Molecular Systematics.2nded.Sunderland,Massachusetts:Sinauer Associates,Inc.,655.
Huelsenbeck JP,Hillis DM.1993.Success of phylogenetic methods in the four-taxon case.Systematic Biology,42(3):247-264.
Huelsenbeck JP,Ronquist F.2001.MRBAYES:Bayesian inference of phylogenetic trees.Bioinformatics,17(8):754-755.
Inger RF,Zhao EM,Shaffer HB,Wu GF.1990.Report on a collection of amphibians and reptiles from Sichuan,China.Fieldiana Zoology,58:1-24.Ivanova NV,Dewaard JR,HebertPDN.2006.An inexpensive,automation-friendly protocol for recovering high-quality DNA.Molecular Ecology Notes,6(4):998-1002.
Jobb G,VonHaeselerA,StrimmerK.2004.TREEFINDER:a powerful graphical analysis environment for molecular phylogenetics.BMC Evolutionary Biology,4:18.
Kumar S,Stecher G,Tamura K.2016.MEGA7:molecular evolutionary genetics analysis version 7.0 for bigger datasets.Molecular Biology and Evolution,33(7):1870-1874.
Lim LJ.1998.The taxonomy of West Malaysian and Singapore Scincidae(Reptilia:Sauria).Master Thesis,National University of Singapore.
Luu QV,Nguyen QT,Pham TC,Dang NK,Vu NT,Miskovic S,Bonkowski M,Ziegler T.2013.No end in sight?Further new records of amphibians and reptiles from Phong Nha-Ke Bang National Park,Quang Binh Province,Vietnam.Biodiversity Journal,4(2):285-300.
Mittleman MB.1950.The generic status ofScincus lateralisSay,1823.Herpetologica,6(2):17-20.
Murphy RW,Crawford AJ,Bauer AM,Che J,Donnellan SC,Fritz U,Haddad CFB,Nagy ZT,Poyarkov NA,Vences M,Wang WZ,Zhang YP.2013.Cold Code:the global initiative to DNA barcode amphibians and nonavian reptiles.Molecular Ecology Resources,13(2):161-167.
Nagy ZT,Sonet G,Glaw F,Vences M.2012.First Large-Scale DNA Barcoding Assessment of Reptiles in the Biodiversity Hotspot of Madagascar,based on Newly Designed COI Primers.PLoS One,7(3):e34506.
Nazarov R,Poyarkov NA,Orlov NL,Phung TM,Nguyen TT,Hoang DM,Ziegler T.2012.Two new cryptic species of theCyrtodactylus irregulariscomplex(Squamata:Gekkonidae)from southern Vietnam.Zootaxa,3302:1-24.
Nazarov RA,Poyarkov NA,Orlov NL,Nguyen NS,Milto KD,Martynov AA,Konstantinov EL,Chulisov AS.2014.A review of genusCyrtodactylus(Reptilia:Sauria:Gekkonidae)in fauna of Laos with description of four new species.Proceedings of the Zoological Institute Russian Academy of Sciences,318(4):391-423.
Neang T,Grismer LL,Chan KO,Grismer JL,Wood Jr PL,Youmans TM.2010.First report of the herpetofauna of Dalai Mountain in Phnom samkos wildlife sanctuary,southwestern Cardamom Mountains,Cambodia.Cambodian Journal of Natural History,2010(2):127-143.
Neang T,Grismer LL,Hun S,Phan C.2015.New herpetofauna records and range extensions forDaboia siamensis(Smith,1917)andGekko petricolusTaylor,1962 from Cambodia.Cambodian Journal of Natural History,2015(2):172-182.
Neang T,Poyarkov NA.2016.First record of the Buonluoi Forest SkinkSphenomorphus buenloicusDarevsky&Nguyen,1983(Squamata:Scincincidae)from Cambodia.Cambodian Journal of Natural History,2016(2):114-118.
Nguyen TQ,Nguyen SV,B?hme W,Ziegler T.2010a.A new species ofScincella(Squamata:Scincidae)from Vietnam.Folia Zoologica,59(2):115-121.
Nguyen TQ,Ananjeva NB,Orlov NL,Rybaltovsky E,B?hme W.2010b.A new species of the genusScincellaMittlemann,1950(Squamata:Scincidae)from Vietnam.Russian Journal of Herpetology,17(4):269-274.
Nguyen TQ,Nguyen TT,Orlov NL.2010c.New record of the Mountain ground skinkScincella monticola(Schmidt,1925)(Squamata:Scincidae)from Cao Bang Province,Vietnam.Herpetology Notes,3(1):201-203.
Nguyen QT,Schmitz A,Nguyen TT,Orlov NL,B?hme W,Ziegler T.2011.Review of the genusSphenomorphusFitzinger,1843(Squamata:Sauria:Scincidae)in Vietnam,with description of a new species from northern Vietnam and southern China and the fi rst record ofSphenomorphus mimicusTaylor,1962 from Vietnam.Journal of Herpetology,45(2):145-154.
Nguyen SN,Le TNT,Tran TAD,Orlov NL,Lathrop A,Macculloch RD,Le TDT,Jin JQ,Nguyen LT,Nguyen TT,Hoang DD,Che J,Murphy RW,Zhang YP.2013.Phylogeny of theCyrtodactylus irregularisspecies complex(Squamata:Gekkonidae)from Vietnam with the description of two new species.Zootaxa,3737(4):399-414.
NuttallM,DimentA,Silverman J,Yeang D.2015.Information brief-monitoring biodiversity co-bene fi ts in the seima protection forest REDD+Demonstration site Mondulkiri and Kratie Provinces,Cambodia.Internal Report to WCS.Cambodia:REDD.
Orlova VF,Poyarkov NA,Chirikova MA,Nazarov RA,Munkhbayar M,Munkhbayar K,Terbish K.2017.MtDNA differentiation and taxonomy of Central Asian racerunners ofEremias multiocellata-E.przewalskiispecies complex(Squamata,Lacertidae).Zootaxa,4282(1):1-42.
Ouboter PE.1986.A revision of the genusScincella(Reptilia:Sauria:Scincidae)ofAsia,with somenoteson itsevolution.Zoologische Verhandelingen,229(1):1-66.
Pham VA,Le DT,Nguyen SLH,Ziegler T,Nguyen TQ.2015.New provincial records of skinks(Squamata: Scincidae)from northwestern Vietnam.Biodiversity Data Journal,3:e4284.
Posada D,Crandall KA.1998.Modeltest:testing the model of DNA substitution.Bioinformatics,14(9):817-818.
Poyarkov NA Jr,Vassilieva AB,Orlov NL,Galoyan EA,Tran TAD,Le DTT,Kretova VD,Geissler P.2014.Taxonomy and distribution of narrow-mouth frogs of the genusMicrohylaTschudi,1838 (Anura: Microhylidae)from Vietnam with descriptions of five new species.Russian Journal of Herpetology,21(2):89-148.
Poyarkov NA Jr,Orlov NL,Moiseeva AV,Pawangkhanant P,Ruangsuwan T,Vassilieva AB,Galoyan EA,Nguyen TT,Gogoleva SS.2015a.Sorting out moss frogs:mtDNA data on taxonomic diversity and phylogenetic relationships of the Indochinese species of the genusTheloderma(Anura,Rhacophoridae).Russian Journal of Herpetology,22(4):241-280.
Poyarkov NA Jr,Rowley JJL,Gogoleva SS,Vassilieva AB,Galoyan EA,Orlov NL.2015b.A new species ofLeptolalax(Anura:Megophryidae)from the western Langbian Plateau,southern Vietnam.Zootaxa,3931(2):221-252.Poyarkov NA Jr,Duong TV,Orlov NL,Gogoleva SS,Vassilieva AB,Nguyen LT,Nguyen VHD,Nguyen SN,Che J,Mahony S.2017.Molecular,morphological and acoustic assessment of the genusOphryophryne(Anura,Megophryidae)from Langbian Plateau,southern Vietnam,with description of a new species.ZooKeys,672:49-120.
Pyron RA,Burbrink FT,Wiens JJ.2013.A phylogeny and revised classification of Squamata,including 4161 species of lizards and snakes.BMC Evolutionary Biology,13:93.
Rambaut A,Drummond AJ.2007.Tracer v1.5.http://beast.bio.ed.ac.uk/Tracer.
Ronquist F,Huelsenbeck JP.2003.MrBayes 3:Bayesian phylogenetic inference under mixed models.Bioinformatics,19(12):1572-1574.
Rowley JJL,Tran DTA,Le DTT,Dau VQ,Peloso PLV,Nguyen TQ,Hoang HD,Nguyen TT,Ziegler T.2016.Five new,microendemic Asian leaf-litter frogs(Leptolalax)from the southern Annamite mountains,Vietnam.Zootaxa,4085(1):63-102.
Sambrook J,Fritsch EF,Maniatis T.1989.Molecular Cloning:A Laboratory Manual.2nded.New York:Cold Spring Harbor Laboratory Press.
Schmidt KP.1925.New reptiles and a new salamander from China.American Museum Novitates,(157):1-5.
Shreve B.1940.Reptiles and amphibians from Burma with descriptions of three new skinks.Proceedings of the New England Zoological Club,18:17-26.
Smith AM,Poyarkov NA Jr,Hebert PDN.2008.CO1 DNA barcoding amphibians:take the chance,meet the challenge.Molecular Ecology Resources,8(2):235-246.
Solovyeva EN,Poyarkov NA,Dunaev EA,Duysebayeva TN,Bannikova AA.2011.Molecular differentiation and taxonomy of the sunwatcher toad-headed agama species complexPhrynocephalussuperspecieshelioscopus(Pallas 1771)(Reptilia:Agamidae).Russian Journal of Genetics,47(7):842-856.Solovyeva EN,Poyarkov NA,Dunayev EA,Nazarov RA,Lebedev VS,Bannikova AA.2014.Phylogenetic relationships and subgeneric taxonomy of toad-headed agamasPhrynocephalus(Reptilia,Squamata,Agamidae)as determined by mitochondrial DNA sequencing.Doklady Biological Sciences,455(1):119-124.(in Russian)
Stuart BL,Emmett DA.2006.A Collection of Amphibians and Reptiles from the Cardamom Mountains,Southwestern Cambodia.Chicago:Field Museum of Natural History,1-27.
Stuart BL,Sok K,Neang T.2006.A collection of amphibians and reptiles from hilly eastern Cambodia.The Raffles Bulletin of Zoology,54(1):129-155.
Stuart BL,Rowley JJL,Neang T,Emmett DA,Som S.2010.Significant new records of amphibians and reptiles from Virachey National Park,northeastern Cambodia.Cambodian Journal of Natural History,(1):38-47.Taylor EW.1963.The lizards of Thailand.University of Kansas Science Bulletin,44(14):687-1077.
Teynié A,David P,Ohler A,Luanglath K.2004.Notes on a collection of Amphibians and Reptiles from Southern Laos,with a discussion of the occurrence of Indo-Malayan species.Hamadryad,29(1):33-62.
Thompson JD,Gibson TJ,Plewniak F,Jeanmougin F,Higgins DG.1997.The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools.Nucleic Acids Research,25(24):4876-4882.
Uetz P,Freed P,Ho?ek J.2018[2018-03-15].The reptile database.http://www.reptile-database.org.
APPENDIX I
Examined materials:
Scincella nigrofasciatasp. nov. (8 specimens): CBC02545-46,CBC02840-45: O’Raing District,Mondulkiri Province,eastern plain,Cambodia.
Scincellamelanosticta(8 specimens): CBC01009,CBC01020-22,CBC01430,CBC01434,CBC01450,CBC01454:Phnom Samkos Wildlife Sanctuary,Veal Veng District,Pursat Province,Cardamom Mountains,Cambodia.
Scincella reevesii(9 specimens):CBC01357-58,CBC01149,CBC01342,CBC01379-80,CBC01382,CBC01479,CBC02305:Phnom Samkos Wildlife Sanctuary,Cardamom Mountains,southwest Cambodia.
Scincella rupicola(10 specimens):CBC02407-10,CBC02412-15:Phnom Chi,Sandan District,Kampong Thom Province,Prey Lang;CBC02323,CBC02339:Karst,Thalavorivath District,Stung Treng Province,northern Prey Lang.
Scincella rufocaudata:holotype ofSphenomorphus rufocaudatus(Darevsky&Nguyen,1983):ZISP 19797 Coll.Darevsky I.S.16-21.06.1983,Buon Luoi,Kon Tum-Gia Lai Province(now in Gia Lai Province),Vietnam.
- Zoological Research的其它文章
- A new karst-dwelling bent-toed gecko(Squamata:Gekkonidae:Cyrtodactylus)from Xiangkhoang Province,northeastern Laos
- A new species of the genus Leptolalax(Anura:Megophryidae)from southern Vietnam
- A new species of the genus Theloderma Tschudi,1838(Amphibia:Anura:Rhacophoridae)from Tay Nguyen Plateau,central Vietnam
- A new genus and three new species of miniaturized microhylid frogs from Indochina(Amphibia:Anura:Microhylidae:Asterophryinae)

