Synergistic renoprotective effect of a compiled branched-chain amino acids and Cymbopogon schoenanthus extract against experimentally induced oxido-nitrosative renal insult
Omar Abdel-Hamed Ahmed-Farid, Bosy Azmy Abd El-Motelp, Enaam Abdel-Mohsen Essa,Mohamad Warda
1Physiology Department, National Organization for Drug Control and Research, Giza 12553, Egypt
2Zoology Department-Faculty of Women for Arts, Science and Education, Ain Shams University, Asmaa Fahmy Street Heliopolis, 11566 Cairo, Egypt
3Department of Biochemistry and Chemistry of Nutrition, Faculty of Veterinary Medicine, Cairo University, Giza 12211, Egypt
1. Introduction
Environmental pollution is a global problem with increasing concern due to blasting growth of industrialization. For decades,the hexavalent chromium (chromium Ⅵ) is a common air born and groundwater pollutant with over hundreds of tons annual production[1]. Chromium salt is used in different industrial applications (U.S. Department of the Interior and U.S. Geological Survey; Mineral Commodity Summaries 2004). Its air pollution effect is a previously unrecognized risk for kidney disease; however,it is recently accused of kidney disease progression. The increased concentration of the fine particulate matter with an aerodynamic diameter (less than 2.5 μm) potentiates the risk of incident chronic kidney diseases by mechanical hindrance of glomerular filtration rate with consequent end-stage renal disease[2]. Former investigation on animal models showed that prolonged exposure for these particles provokes oxidative stress and inflammation with altered renal hemodynamics[3]. Moreover,the soluble chromium found in leather tanning, groundwater, and industrial waste is recognized as an environmental contaminant with teratogenic mutagen and potent nephrotoxic pollutant[4].
On molecular basis, the chromium Ⅵ has a structural similarity to other circulating sulfate and phosphate anions. This facilitates its cellular entry via competition for their non-specific anion channels[5,6]. On entry, extra damage would occur during its reduction via depleting cellular anti-oxidant reserve, e.g., active thiol-containing glutathione. After reduction, chromium seeks the formation of a thermodynamically stable adduct with cellular nucleophiles like DNA and other proteins. These events result in increased oxidative stress with disrupted cellular redox potential,double DNA breakage manifested by elevated 8-hydroxy-2-deoxyguanosine (8OHdG) level and marked denaturation of vulnerable cellular proteins and perturbed cellular membranes[7].Although the chromate is recently proved to induce nephrotoxic cellular damage, the study on amelioration of its pro-oxidant renal insult is still in its infancy[8].
The branched-chain amino acids (BCAAs), such as valine, leucine,and isoleucine, are indispensable amino acids. Their altered plasma profile is the common finding of de-compensated renal failure with blood acidosis[9]. BCAAs metabolic disturbance inflicts cellular performance especially in organs with high protein and energy turnover. The kidney plays a key role in acid-base balance with major contribution in metabolic homeostasis[10]. The renal regenerative capacity is consequently improved with enhanced protein synthetic machinery. BCAAs cope with local oxidative stress[11] and participate in protein synthesis by activating a specific mammalian kinase that stimulates eukaryotic initiation factor 4E and enhancement of polysomes enrichment[12]. Moreover, renal parenchyma is highly dependent on BCAAs because of its high BCAA-transferase and low branched-chain keto-acid-dehydrogenase activities[11]. Despite the large body of evidence concerning the regenerative capacity of BCAAs[13], their actual role on ameliorating the heavy metal-nephritic insult in lab model is not fully recovered.The herbal extract, as an alternative medicine, exerts favorable neutralizing effect against toxin-including drugs, pollutants, and chemicals. Many herbal extracts have an antioxidant protective effect[14]. Their observed mild action could explain their current blasting use in parallel with their synthetic pharmaceutical counterparts. Cymbopogon schoenanthus (CS) has antioxidant, antiinflammatory, detoxification, and chemoprotective properties[15].The fresh leaves of CS are widely used as a traditional medicine in Arab peninsula. While inducing hepatotropic effect, oral intake of their water extract is believed as a potent remedy against kidney stones in North African arid Sahara[16]. Their actual guardian effect against the deleterious influence of PDC as a potent nephrotoxic pollutant in animal lab model is still underestimated. Therefore, this study was adopted to evaluate the localized regenerative capacity of BCAAs as well as the possible defensive role of CS extract during renal PDC-induced nephrotoxicity in lab animal model. Furthermore,their compiled possible synergistic action was investigated against this kind of renal insult for the first time.
2. Materials and methods
2.1. Rats
Adult male Sprague-Dawley rats weighting (220-250 g) from NODCAR animal house (Egypt) were maintained at (27±1) ℃with equal daily dark/light cycle. They had free access to water and food (standard pellet chow) during the whole experimental period. The experiments were carried out between 12:00 and 15:00 with a previously approved protocol[17] and in accordance with the guideline standards of the experimental animals' treatment at the Faculty of Veterinary Medicine, Cairo University, Egypt.
2.2. Chemicals
Methanol (HPLC grade) were purchased from Loba Co, India.Perchloric acid were purchased from Loba Co, India. Sulphosalsilic acid and P-aminobenzyl glutamate and pyrogallol were purchased from TMMEDIA Co, India. The 1,1,3,3-tetraethoxypropane,glutathione (GSH) and oxidized glutathione (GSSG) from Sigma Aldrich (USA). Potassium dichromate (PDC), monobasic potassium phosphate, nitrites and nitrate were from Al Nasr chemicals,Abozabal (Qualybia, Egypt). All used chemicals were either HPLC or analytical grade.
2.3. Preparation of extract
Approximately 50 g of CS leaves were soaked with 10 volumes of 70% ethanol for 3 d. Extraction was performed by 40-minute water bath sonication. The content was then filtrated via Whatman filter paper (Bibby RE200, Sterilin Ltd., UK) prior to hot drying at 45 ℃to obtain the gummy extract[18].
2.4. Grouping of animals
Animals were divided into five equal groups (n=6) with the following treatments: Group 1 served as control. Group 2 was the treated modeling group with PDC intra-peritoneal injection(15 mg/kg b.w) according to Biber[19]. Group 3 was with an oral administration of BCAAs for 15 d prior to PDC injection (116,76, 76 mg/kg b.w. for leucine, isoleucine, and valine respectively),according to Smriga[20]. Group 4 was with an oral administration of CS ethanolic leaves extract (200 mg/kg b.w.) for 15 d prior to PDC injection[15]. Group 5 was with mixed oral administration of BCAA and CS for 15 d before PDC injection.
The experiment lasted for 17 d designed for prophylactic treatment for 15 d and then 2 d after induction of nephrotoxicity by PDC. At the end of the experiments (day 18) animals were slaughtered to get tissue samples.
2.5. Blood collection
At the end of the experiment, blood samples were collected and serum samples were obtained after centrifugation at 3 000 r/min for 20 min and kept at -20 ℃ until further analysis. Other blood samples were collected in heparinized tubes for determination of hemoglobin and centrifuged (3 000 r/min for 30 min) to obtain plasma for total plasma protein determination.
2.6. Preparation of kidney homogenates
Rats were sacrificed and kidneys were collected and then washed with cooled saline (0.9%). The harvested kidney samples were divided for biochemical assays [malonaldehyde (MDA), GSH,GSSG, superoxide dismutase (SOD), 8OHdG, tumor necrosis factor α (TNF-α), myeloperoxidase (MPO), nitric oxide (NO),and interleukin-1β (IL-1β)] and histological examinations. The kidney tissues under investigation were homogenized with icecold phosphate buffer saline (pH 7.4) using Potter-Elvehjem glass homogenizer to prepare 10% (w/v) homogenate. The particulate debris was removed by 5 000 r/min centrifugation for 20 min at 4 ℃ (Sigma-3K30, Germany) and the clear resultant supernatant was used for different enzyme assays.
2.7. Determination of biochemical parameters
Urea, creatinine, total protein, hemoglobin, ferritin, iron,sodium, potassium, calcium, and phosphorus were determined with colorimetric method by spectrophotometer according to kit procedure (Spectrum, Egyptian company of biotechnology-Egypt).The biochemical parameters were determined by spectrophotometer(UV-Visible spectrophotometer, Shimadzu 2450 Kiyamachi Nijo Minami, Nakagyo-kU, Japan).
2.7.1. Determination of MDA by HPLC
HPLC standardization condition for MDA detection utilized Agilent instrument equipped with Supelcosil C18 column and the flow rate of 1.5 mL/min. The 250 nm wave length detection was performed using standard 1,1,3,3 tetraethoxypropane solution and phosphate buffer (3.6 pH): methanol (82.5:17.5) as previously stated[21]. Kidney tissue preparation for MDA determination by HPLC followed our previous work[22].
2.7.2. Determination of nitrites and nitrates by HPLC
Nitrites and nitrate were determined according to the method of Papadoyannis et al[23] by HPLC. A standard mixture of nitrite and nitrate was used to determine the retention times and separation of the peaks. The samples were resolved by anion exchange (PRPX100 Hamilton, 150 mm × 4.1 mm, 10 μm) analytical column using 0.1 mol/L NaCl:methanol (45:55, v/v) mobile phase with 2 mL/min flow rate and 230 nm wavelength detection limit.
2.7.3. Determination of GSH and GSSG by HPLC
The concentration of reduced or oxidized glutathione was measured by HPLC-tracing of their thiol groups after the method of Jayatilleke and Shaw[24] using reduced and oxidized glutathione reference standards (1 mg/mL in 70% methanol).
2.7.4. Determination of kidney 8-OHdG
Determination of 8-OHdG was determined in kidney tissue homogenates by HPLC according to our previous study of Abd-Elrazek and Ahmed-Farid[25] against standard solution of 8OHdG using Supelcosil C18 column and phosphate buffer mobile phase(50 mm KH2PO4in water:methanol (85:15, v/v) at 5.5 pH) with 0.6 mL/min flow rate and 245 nm detection limit.
2.7.5. Determination of SOD
SOD activity was assayed in the kidney homogenate by the method of Marklund and Marklund[26]. The enzyme activity was expressed as 1 U/g tissue, and enzyme activity is the activity capable for 50%inhibition of pyrogallol auto-oxidation in 1 min.
2.7.6. Determination of inflammatory mediators
TNF-α, MPO, and IL-1β were determined with ELISA technique according to RayBio?Rat, kits procedure (RayBio, Spain).
2.8. Histopathological examination
Kidney tissue samples were collected. The specimens were prepared and stained with hematoxylin and eosin stains for histopathological examination according to Banchroft et al[27] and visualized using electric light microscope (Optic, CFI60 Infinity,USA).
2.9. Immunohistochemical staining of proliferating cell nuclear antigen
TNF-α polyclonal antibody (Thermo Scientific Co., USA) was used to evaluate renal inflammation parallel with hematoxylin counterstaining. The detail immunohisochemical procedure followed our previous work[28].
2.10. DNA comet assay
The comet assay of DNA in kidney homogenate was estimated according to the classic alkaline single-cell electrophoresis protocol[29]. Samples were stained with ethidium bromide Ⅰ and analyzed by Comet Score 1.5 software. Percent of DNA in comet tails was considered as the marker of genotoxic effect.
2.11. Statistical analysis
Data are expressed as the mean ± S.E.M for the six rats in each group. Statistical differences between groups were evaluated by oneway analysis of variance (ANOVA) using SAS. Statistical analysis of the obtained data was performed using the general linear model(GLM). Significant differences among means were evaluated using Duncan's Multiple Range Test.
3. Results
3.1. Effects of BCAA, CS, and their combination on electrolytes balance and biochemical parameters in rats treated with PDC
The PDC injected group (Table 1) showed a generalized disturbance in all measured serum electrolytes comparable to control(P<0.05) in the form of hypernatremia and hyperphosphatemia with recorded hypokalemia and hypocalcemia. A partial restoration of plasma electrolytes homeostasis was noticed in BCAAs and CS treated groups. The compiled administration of BCAAs and CS,however, restored the plasma electrolytes to its normal control values(Table 1). The observed serum electrolytes disturbance was tightly coherent with other organ dysfunction. It is clear from the result that the kidney insult induced by PDC (15 mg/kg b.w) altered blood chemistry in the form of elevated levels of serum creatinine and blood urea nitrogen (Table 1) with an evidence of renal insufficiency rather than liver dysfunction. Maximal amelioration of this alteration was observed in the group with compiled BCAAs and CS administration where the level of both urea and creatinine decreased to near normal values. The separate administration of either BCAAs or CS extraction induced a non-significant decrease in their blood values than that in PDC group but the blood values were still higher than control group. The data in Table 1 showed a significant decrease in total protein and oxygen carrying capacity in PDC group when compared with the control. This decrease was restored to normal values after compiled BCAAs and CS extraction treatment.
3.2. Effects of BCAA, CS, and their combination on inflammatory mediators in rats treated with PDC
The elevated serum creatinine and blood urea nitrogen turned our attention for further close screening of localized renal affections.It is clear from Table 2 that PDC-induced renal insult caused a marked decrease (P<0.05) in both GSH and SOD contents in kidney tissue. These recorded decreases were significantly improved in CS and BCAAs+CS groups. The PDC-induced renal insult not only deviated the redox homeostasis in the inflicted tissues but further sabotaged the cellular performance as seen from oxido-nitrosative membrane peroxidation (e.g., increased levels of NO and MDA)with accelerated apoptosis accounted by an increase in 8OHdG and proved by the comet assay. The data, however, display that after treatment with BCAAs or CS extract or their combined treatment significantly (P<0.05) restored the NO and partially restored MDA,GSH, GSSG and 8OHdG levels to their original values in control group.
3.3. Effects of BCAA, CS, and their combination on oxidative stress markers in rats treated with PDC
The result of this study showed that in PDC treated rats a recognized increase (P<0.05) in inflammatory mediators levels(Table 2). While the BCAAs treatment significantly restored the level of MPO (P<0.05) to its original normal value in control kidney tissue; however, other treatments partially restored this value. This effect was potent with BCAAs administration and their combination with CS extract.
The obtained biochemical data were extra-confirmed by microscopic evidences. As seen from Figure 1, the PDC induced congestion in glomerular capillaries with edema and necrosis in renal tubular epithelium comparable with control. The BCAAs treated group showed dilatation tubules and mild congestion. The CS group showed a slight congestion in glomerular capillaries with negligible necrosis in renal tubular epithelium. Renal tissue of compiled BCAAs and CS treatment group showed much restoration in nephron morphology. To prove the microscopic finding of increased inflammatory response, the anti-TNF-α immune-staining in nephrons after different treatments (Figure 2) showed the strong positivity against TNF-α expression in PDC-insult was markedly attenuated after BCAAs treatment and completely abolished aftercompiled BCAAs and CS treatment. The accelerated apoptosis was confirmed by the comet assay (Figure 3 & Table 3), where the higher comet score presented after PDC-renal insult disappeared after the compiled treatment with BCAAs and CS.
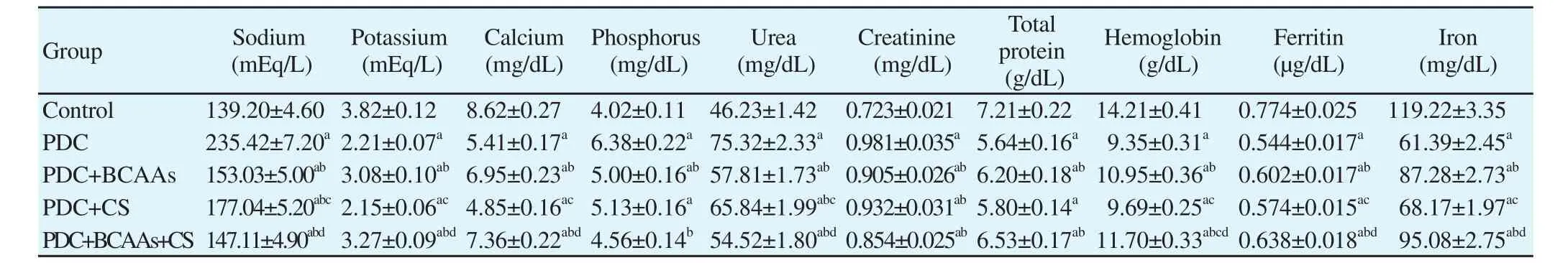
Table 1 Effects of branched chain amino acids (BCAAs), Cymbopogon schoenanthus (CS), and their combination on kidney function in rats treated with potassium dichromate (PDC).
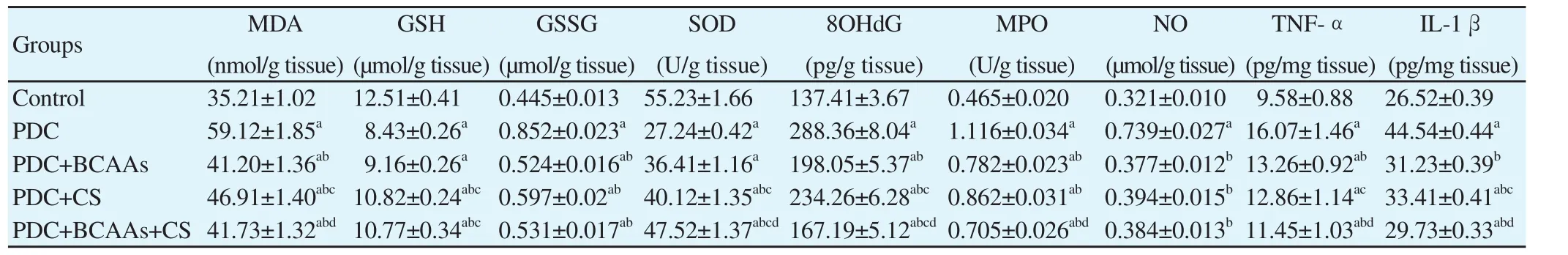
Table 2 Effects of branched chain amino acids (BCAAs), Cymbopogon schoenanthus (CS), and their combination on kidney function and inflammatory mediators levels in rats treated with potassium dichromate (PDC).
4. Discussion
The exposure to hexavalent chromium (chromium Ⅵ) compounds has an increasingly global concern. For decades, they are definedas potent multivalent toxins and carcinogens. They could induce nephrotoxicity, since these pro-oxidants are easily absorbed and diffusible across cell membrane with strong oxidative potential. The non-specific sulfate anion channels are the main route of entry where chromium ions compete with their similar charged sulfates[5,6].
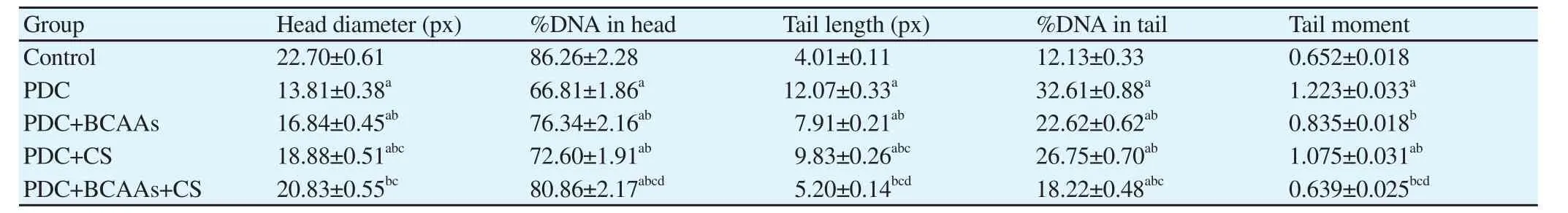
Table 3 Effects of branched chain amino acids (BCAAs), Cymbopogon schoenanthus (CS), and their combination on kidney comet assay in rats treated with potassium dichromate (PDC).
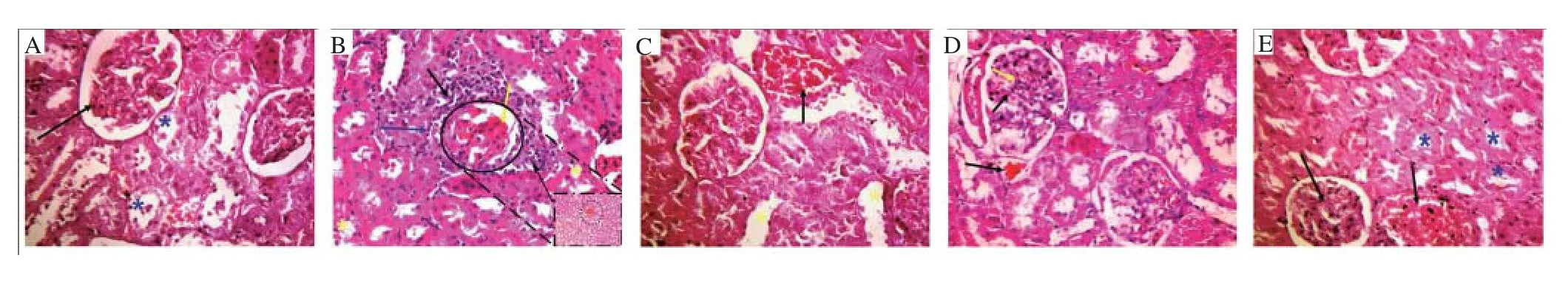
Figure 1. Hematoxilin-eosin stained micrographs of renal tissue at the end of the experiment (×400).

Figure 2. Micrographs of renal tissue showing anti-TNF-α immune-staining after 17 d prophylactic doses against PDC-induced nephrotoxicity (×400).

Figure 3. Comet images of renal tissue cells obtained from all groups after 17 d prophylactic doses against PDC-induced nephrotoxicity.
This followed by a concomitant massive reactive oxygen speices production during their intracellular reduction, which is the main characteristic of chromium Ⅵ metabolism[5]. The reactive oxygen speices gush could induce renal tubular damage[30] with MDA flooding[31] and exhaustion of antioxidant markers, e.g., GSH and SOD. Meanwhile, chromium Ⅵ ion induces high oxidative damage to proteins and other biomolecules. Previous studies proved that chromium Ⅵ activates several mitogens-like protein kinases extracellular regulated protein kinases-1, extracellular regulated protein kinases-2, c-Jun N-terminal kinase and p38[32] with enhanced phosphorylation of nuclear factor κB transcription factor. Indeed the activation of nuclear factor κB triggers various inflammatory mediators[33]. An increasing body of evidence suggests the common link between disrupted metabolism with cellular immune response and inflammation[34]. Our obtained data explain, in part, the observed global disrupted homeostasis in our study after PDC-intoxication in the form of altered blood biochemical parameters. The decreased hemoglobin concentration together with lowering of serum iron,total protein and serum ferritin in PDC logically implies a decreased oxygen saturation capacity with a state of global hypoxia. An earlier related study demonstrated that chromium may compete with iron for apo-transferrin binding sites. This kind of competition decreases serum iron and badly influences iron metabolic role[35]. The elevated blood urea nitrogen and serum creatinine levels, on the other hands,clearly indicate the renal insult rather than liver damage. This renal insult was proved microscopically on the nephron level by defected filtration power evidenced by shrunken glomerular tuft epithelium with necrosis and disturbed reabsorption capacity manifested by the congested peritubular capillaries with tubular dilation and loss of brush borders. The increased level of inflammatory mediators,e.g., myeloperoxidase activity and IL-1β level with TNF-α over-expression in glomerular capillaries and proximal tubules,announces a complicated inflammatory infiltration. Nevertheless,the nitro-oxidative nature of this damage was clearly deduced from the increased MDA and NO levels with collapsed renal antioxidant activities signified by GSH depletion and mitigated SOD activity.Like the previous study on acute intoxication[36], the long-term chromium renal intoxication during this investigation demonstrated a lowering in GSH/GSSG ratio, which impacts a concomitant decrease in its reductase/peroxidase ratio. Together with the decreased SOD activity, our obtained data confirm the collapsed redox potential with prioritized renal oxidative damage. The cellular oxidative stress culminated further lipo-oxidative membrane damage with elevated renal MDA level. As a final outcome, an accelerated apoptosis could be expected from the increased renal level of 8OHdG together with distorted nuclei after PDC insult. Interestingly, the observed increase in inflammatory mediators is proved to be in positively correlation with lipoxative stress parameters and both of them correlate positively with the increase in blood urea nitrogen.Moreover, serum electrolytes, the real narrators of the renal intracellular story, are also affected. Sodium is the most extracellular cation that plays an important role in maintaining fluid balance. The observed PDC-induced hypernatremia could imply the impaired renal functions with the decreased excretory ability[37]. Other serum ions like calcium and phosphate are indispensable multivalent ions modulating the vast majority of cellular activities. The kidneys play a central role in their homeostasis via complicated ion gated pathways. Their deviated values in the serum of PDC treated group could also support the nation of disrupted renal function. It is worth noting that the observed hypocalcemia might reflect the membrane re-absorption defective function at the proximal convoluted tubules where 80% of the filtered calcium is passively reabsorbed by the ambient electrochemical gradient for calcium[38]. Since calcium ions are potent anti-inflammatory cations, it is logically to find a negative correlation between the decreased serum calcium level and the reported elevation in inflammatory response mediators. Similarly,the decreased level of serum phosphate could largely attribute to a malformation in apical brush border membrane renal proximal tubule where most of the phosphate re-absorption is dependent on its integrity[39].
Yet so far the ability of BCAAs or herbal extract of CS as natural products with therapeutic potential against the pro-oxidative PDC-induced renal-insult is not fully resolved. The benefit of the use of BCAAs in improving cellular regeneration and limiting oxidative stress is a matter of controversy[39,40]. A recent study favored the supplementation with BCAAs as a safe intervention with positive impact in patients with cirrhosis[41]. In addition, BCAA-containing peptides modulated oxidative stress and reduced the severity of fatty liver in an experimental animal model[42]. The decreased blood levels of total protein and ferritin after PDC-insult might be compensated by BCAAs. The increased level of BCAAs, on the other hand, provokes an inflammatory response guarded by prooxidative mediators in cultured leukocytes[43]. This type-dependent discrepancy in cellular behavior acknowledges a peculiarity of response as a function of cellular location. During renal insult,kidney cells have special strategy to compete their energy and regenerative demands from the available BCAAs, where they are extra-hepatically metabolized in tricarboxylic acid cycle to anapleurotic or energy-producing intermediates[44,45].
The value of BCAAs appears to go further in contribution of protein synthesis[46]. L-leucine, a member of BCAAs, activates mammalian protein synthesis via rapamycin (mammalian target of rapamycin) signaling pathway[47]. The up-regulation of albumin synthesis induced by BCAAs administration may be attributed to the accelerated phosphorylation of p70 S6 kinase and 4E-BP1 in livers with promotion of hepatic mammalian target of rapamycin signaling[48]. Therefore, it is logically to notice an improvement in plasma total protein level after BCAAs addition.
These data can explain the role of BCAAs supplementation with or without CS in restoring homeostasis as well as attenuation of PDC-induced nephrotoxicity and renal oxidant stress. Leakage and depletion of filtration function of kidney may be compensated by BCAAs which enhance protein syntheses. As demonstrated from our finding the BCAAs might act as cell membrane protective antioxidant that lead to normalized kidney function and prevent minerals leakage through urine excretion. Antioxidant features of BCAAs come from the enhancement of endogenous antioxidant glutathione synthetase (EC 6.3.2.3) via glutamine amino acid in the presence of cysteine to produce γ glutamyle cysteine prior to conversion of active glutathione when coupled with glycine[49].The present finding is inconsistent with previous data[50] who reported that the BCAAs-rich protein increases the GSH level and decreases MDA in rats under stress. Zemel reported that leucine has an anti-inflammatory action by reduction of oxidative stress and inflammatory mediators[51].
Moreover, cellular increased level of BCAAs in duodenal mucosal side is recently proved to be potent stimulators of the duodenal calcium absorption[52]. This fact adds an advantage to the use of BCAAs to restore normocalcimia and to cope against the decreased serum calcium level after PDC-intoxication.
We screened the use of CS extract as traditional alternative medicine with a potential protective effect against the PDC-induced renal insult. Previous studies reported that the leaves of CS contain saponins, flavonoids and serve as good scavenger against superoxide anions. Interestingly, the saponins as terpene glycosides are recognized recuperative power stimulants with natural resistance improvement[51,53]. Worthwhile, the decreased in oxidative stress markers in our results may attribute to the indirect tannins chelation or direct antioxidant properties of flavonoid. This finding is in agreement with that of Singh et al. who stated that flavonoid has a beneficial effect for removal of urea and creatinine from plasma of normal mice treated with its alcoholic extract and considered as a therapeutic herb to manage renal function[54]. The normalization of total protein, hemoglobin, as well as iron and ferritin level in the CS group may be due to saponin, which was successfully used to promote blood circulation while iron is important in the production of hemoglobin and it plays an important role in flavor protein cytochrome system activities[55]. The correction of serum electrolytes in the CS group could be induced by the steroidal like saponin action that preserves the level of serum cations and enhances their intestinal absorption. Further renoprotective effect of saponin is attributed by inhibition of the intra-renal renin-angiotensin-aldosterone system[56].Normalized of inflammatory mediators may be due to suppression of the production of lipoprotein-induced NO and chemotactic cytokines by down-regulation of reactive oxygen species generation,inducible nitric oxide synthase and cycloxygenase-2 expression[57].A combined BCAAs and CS treatment significantly attenuated renal dysfunction and the observed morphological alterations. The combined treatment also decreased the MDA level and attenuated the reduction of SOD activity in the kidney after PDC-induced renal insult. These data, however, met with the general recovery of blood parameter to normal values. The restored homeostasis was confirmed at the histological level together with a normal return of the level inflammatory mediators and decreased rate of apoptosis.The BCAAs administration markedly reduced the PDC-induced renal DNA damage. Moreover, a recognized restoration of the renal antioxidant capacity with reduced lipid peroxidation was observed after a combined CS and BCAA treatment.
In conclusion, the PDC treatment in rat animal model induced an oxidative renal damage with accelerated local inflammatory response and global alteration in blood parameters. The PDC-induced renal insult was corrected by treatment of either BCAAs or CS. The combined BCAAs and CS treatment has a better renoprotective effect from being separately used. The study warrants against the PDC renal intoxication and affords novel natural product candidates for recovery.
Conflict of interest statement
The authors declare that they have no conflict of interest.
[1] Zhao Y, Wang S, Aunan K, Seip HM, Hao J. Air pollution and lung cancer risks in China-a meta-analysis. Sci Total Environ 2006; 366: 500-513.
[2] Seung H, Kyeong M, Yong LK, Sang L, Jinwoong HK, Young C.Chemical constituents isolated from Paeonia lactiflora roots and their neuroprotective activity against oxidative stress in vitro. J Enz Inhib Med Chem 2009; 25: 1138-1140.
[3] Ozen OA, Kus MA, Kus I, Alkoc OA, Songur A. Protective effect of melatonin against formaldehyde-induced oxidative damage and apoptosis in rat testes: An immunohistochem-ical and biochemical study. Syst Biol Reprod Med 2008; 54: 169-176.
[4] Makker K, Ashok A, Rakesh S. Oxidative stress and male infertility.Indian J Med Res 2009; 129: 357-367.
[5] Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: Nickel, arsenic, and chromium.Chem Res Toxicol 2008; 21(1): 28-44.
[6] Alexander J, Aaseth J. Uptake of chromate in human red blood cells and isolated rat liver cells: The role of the anion carrier. Analyst 1995; 120:931-933.
[7] Hofer T, M?ller L. Reduction of oxidation during the preparation of DNA and analysis of 8-hydroxy-2‘-deoxyguanosine. Chem Res Toxicol 1998; 11(8): 882-887.
[8] Swaran J, Flora S, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health 2010; 7(7): 2745-2788.
[9] Nakano M, Nakashima A, Nagano T, Ishikawa S, Kikkawa U, Kamada S. Branched chain amino acids enhance premature senescence through mammalian target of rapamycin complex Ⅰ-mediated upregulation of p21 protein. PLoS One 2013; 8(11): e80411.
[10] Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M.Branched-chain amino acids as a protein and energy source in liver cirrhosis. Biochem Biophys Res Commun 2004; 313(2): 405-409.
[11] Yesilbursa D, Serdar Z, Serdar A, Sarac M, Jale C. Lipid peroxides in obese patients and effects of weight loss with orbital on lipid peroxides levels. Int J Obesity 2005; 29: 142-145.
[12] Shveygert M, Kaiser C, Shelton Bradrick S, Gromeier M. Regulation of eukaryotic initiation factor 4E (eIF4E) phosphorylation by mitogenactivated protein kinase occurs through modulation of Mnk1-eIF4G interaction. Mol Cell Biol 2010; 30(21): 5160-5167.
[13] Pereira MG, Baptista IL, Carlassara EO, Moriscot AS, Aoki MS,Miyabara EH. Leucine supplementation improves skeletal muscle regeneration after cryolesion in rats. PLoS One 2014; 9(1): e85283.
[14] Haredy SA, Imam TS, Ahmed-Farid OA. Combination of Ficus carica leaves extract and ubiquinone in a chronic model of lithium induce reproductive toxicity in rats: Hindrance of oxidative stress and apoptotic marker of sperm cell degradation. J Pharm Biolog Sci 2017; 12(II): 64-73.
[15] Al Haznawi AM, Attar AS, Abdulshakoor AA, Ramadan MA. Inhibition of calcium oxalate nephrotoxicity with Cymbopogon schoenanthus (Al-Ethkher). Jazan: Jazan University; 2007.
[16] Al-Ghamdi SS, Al-Ghamdi AA, Shammah AA. Inhibition of calcium oxalate nephrotoxicity with Cymbopogon schoenanthus (Al-Ethkher).Drug Metab Lett 2007; 1(4): 241-244.
[17] Olfert ED. Ethics of animal models of neurological diseases. In: Boulton AA, Baker GB, Butterworth RF, editors. Neuromethods: Animal models of neurological disease, I. Clifton: Humana Press; 1992: p. 1-28.
[18] Algohary AM, Ahmad-Farid OA, Abd-Elrazek AM, Al-Baradie RS.Neuroprotective effects of herbal cocktail on cerebrovascular dysfunction in rats with induced hyperhomocysteinaemia. Biomed Res Ther 2016;3(12): 1045-1061.
[19] Biber TU, Mylle M. Bains AD, Gottschalk CW, Oliver JR, MacDowell MC. A study by micropuncture and microdissection of acute renal damage in rats. Am J Med 1968; 44: 664-705.
[20] Smriga M, Kameishi M, Tanaka T, Kondoh T, Torii K. Preference for a solution of branched-chain amino acids plus glutamine and arginine correlates with free running activity in rats: Involvement of serotonergicdependent processes of lateral hypothalamus. Nutr Neurosci 2002; 5: 189-199.
[21] Karalas F, Karatepe M, Baysar A. Determination of free malondialdehyde in human serum by high performance liquid chromatography. Anal Biochem 2002; 311: 76-79.
[22] Arafa NM, Salem SM, Ahmed-Farid OA. Influence of Echinacea extract pre-or postnatal supplementation on immune and oxidative status of growing rabbits. Ital J Anim Sci 2010; 9: e63
[23] Papadoyannis IN, Samanidou VF, Nitsos CC. Simultaneous determination of nitrite and nitrate in drinking water and human serum by high performance anion-exchange chromatography and UV detection.J Liq Chromatogr R T 1999; 22(13): 2023-2041.
[24] Jayatilleke E, Shaw S. A high performance liquid chromatographic assay for reduced and oxidized glutathione in biological samples. Anal Biochem 1993; 214(2): 452-457.
[25] Abd-Elrazek AM, Ahmed-Farid OA. Protective effect of L-carnitine and L-arginine against busulfan-induced oligospermia in adult rat. Andrologia 2017; 50(1): doi: 10.1111/and.12806.
[26] Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974; 47: 469-474.
[27] Banchroft JD, Stevens A, Turner DR. Theory and practice of histological techniques. 4thed. New York: Churchill Livingstone Publisher; 1996.
[28] Montasser AO, Saleh H, Ahmed-Farid OA, Saad AM, Marie MA.Protective effects of Balanites aegyptiaca extract, melatonin and ursodeoxycholic acid against hepatotoxicity induced by methotrexate in male rats. Asian Pac J Trop Med 2017; 10(6): 557-565.
[29] Xu XR, Zhu JQ, Ye T, Wang CL, Zhu YF, Dahms HU, et al. Improvement of single-cell gel electrophoresis (SCGE) alkaline comet assay. Aquat Biol 2013; 18: 293-295.
[30] Ye J, Li J, Xia R, Zhou M, Yu L. Prohibitin protects proximal tubule epithelial cells against oxidative injury through mitochondrial pathways.Free Radic Res 2015; 49(11): 1393-1403.
[31] Cavdar Z, Ural C, Celik A, Arslan S, Terzioglu G, Ozbal S, et al.Protective effects of taurine against renal ischemia/reperfusion injury in rats by inhibition of gelatinases, MMP-2 and MMP-9, and p38 mitogenactivated protein kinase signaling. Biotech Histochem 2017; 12: 1-12.
[32] Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 2004; 68(2): 320-344.
[33] Chuang SM, Wang IC, Yang JL. Roles of JNK, p38 and ERK mitogenactivated protein kinases in the growth inhibition and apoptosis induced by cadmium. Carcinogenesis 2000; 21: 1423-1432.
[34] Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 2008; 8: 923-934.
[35] Ani M, Moshtaghie AA. The effect of chromium on parameters related to iron metabolism. Biol Trace Elem Res 1992; 32: 57-64.
[36] Kotyzová D, Hodková A, Bludovská M, Eybl V. Effect of chromium (Ⅵ)exposure on antioxidant defense status and trace element homeostasis in acute experiment in rat. Toxicol Ind Health 2015; 31(11): 1044-1050.
[37] Luczak MW, Green SE, Zhitkovich A. Different ATM signaling in response to chromium(Ⅵ) metabolism via ascorbate and nonascorbate reduction: Implications for in vitro models and toxicogenomics. Environ Health Perspect 2016; 124(1): 61-66.
[38] Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 2014; 10(7): 1257-1272.
[39] Lederer E. Renal phosphate transporters. Curr Opin Nephrol Hypertens 2014; 23(5): 502-506.
[40] Yamamoto J, Nishio S, Hattanda F, Nakazawa D, Kimura T, Sata M, et al. Branched-chain amino acids enhance cyst development in autosomal dominant polycystic kidney disease. Kidney Int 2017; 92(2): 377-387.
[41] Ruiz-Margáin A, Macías-Rodríguez RU, Ríos-Torres SL, Román-Calleja BM, Méndez-Guerrero O, Rodríguez-Córdova P, et al. Effect of a highprotein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Rev Gastroenterol Mex 2017; 83(1): 9-15.
[42] Kawaguchi T, Ueno T, Nogata Y, Hayakawa M, Koga H, Torimura T.Wheat-bran autolytic peptides containing a branched-chain amino acid attenuate non-alcoholic steatohepatitis via the suppression of oxidative stress and the upregulation of AMPK/ACC in high-fat diet-fed mice. Int J Mol Med 2017; 39(2): 407-414.
[43] Zhenyukh O, Civantos E, Ruiz-Ortega M, Sánchez MS, Vázquez C, Peiró C, et al. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med 2017;104: 165-177.
[44] O'Connell TM. The complex role of branched chain amino acids in diabetes and cancer. Metabolites 2013; 3(4): 931-945
[45] Lehninger AL, Nelson DL, Cox MM. Lehninger principles of biochemistry.5thed. New York: W.H. Freeman Publisher; 2005.
[46] Sato S, Watanabe A, Muto Y, Suzuki K, Kato A, Moriwaki H, et al. Clinical comparison of branched-chain amino acid (l-Leucine,l-Isoleucine, l-Valine) granules and oral nutrition for hepatic insufficiency in patients with decompensated liver cirrhosis (LIV-EN study). Hepatol Res 2005; 31: 232-240.
[47] Han B, Tong J, Zhu MJ, Ma C, Du M. Insulin-like growth factor-1(IGF-1) and leucine activate pig myogenic satellite cells through mammalian target of rapamycin (mTOR) pathway. Mol Reprod Dev 2008;75(5): 810-817.
[48] Ijichi C, Matsumura T, Tsuji T, Eto Y. Branched-chain amino acids promote albumin synthesis in rat primary hepatocytes through the mTOR signal transduction system. Biochem Biophys Res Commun 2003; 303: 59-64.
[49] Hartwig H, Drechsler M, Lievens D. Platelet-derived PF4 reduces neutrophil apoptosis following arterial occlusion. Thromb Haemost 2013;111(3): 562-564.
[50] Omotade IO. Chemical profile and antimicrobial activity of Cymbopogon citratus leaves. J Nat Prod 2009; 2: 98-103.
[51] Zemel MB. Effects of dairy compared with soy on oxidative and inflammatory stress in overweight and obese subjects. Am J Clin Nutr 2010; 91: 16-22.
[52] Thammayon N, Wongdee K, Lertsuwan K, Suntornsaratoon P,Thongbunchoo J, Krishnamra N, et al. Na+/H+exchanger 3 inhibitor diminishes the amino-acid-enhanced transepithelial calciumtransport across the rat duodenum. Amino acids 2017; 49(4): 725-734.
[53] Ahmed RF, Eldenshary ES, Nada SA, Asaad GF, Arafa NS, Ahme-Farid OA. Pharmacological study of the possible antidepressant activity of whey protein isolate in mice. Aust J Basic Appl Sci 2011; 5(12): 2649-2659.
[54] Singh N, Verma P, Misharu N, Nath R. A comparative evaluation of some anti stress agents of plant origin. Indian J Pharmacol 1991; 21: 99.
[55] Mehrdad M, Messripour M, Ghobadipour M. The effect of ginger extract on blood urea nitrogen and creatinine in mice. Pak J Biolog Sci 2007;10(17): 2968-2971.
[56] Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, Jiang Y. Saponins from edible legumes: Chemistry, processing, and health benefits. J Med Food 2004; 7: 67-78.
[57] Chen M, Long Z, Wang Y, Liu J, Pian H, Wang L, et al. Protective effects of saponin on a hypertension target organ in spontaneously hypertensive rats. Exp Ther Med 2013; 5(2): 429-432.
 Asian Pacific Journal of Tropical Medicine2018年5期
Asian Pacific Journal of Tropical Medicine2018年5期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Zoonotic leishmaniasis and control in Ethiopia
- Antimicrobial activity of kojic acid from endophytic fungus Colletotrichum gloeosporioides isolated from Sonneratia apetala, a mangrove plant of the Sundarbans
- Adjuvant activity of Pasteurella multocida A strain, Pasteurella multocida B strain and Salmonella typhimurium bacterial DNA on cellular and humoral immunity responses against Pasteurella multocida specific strain infections in Balb/c mice
- Anti-cancer effect of ethylacetate fraction from Orostachys japonicus on HT-29 human colon cancer cells by induction of apoptosis through caspase-dependent signaling pathway
- Melioidosis in India and Bangladesh: A review of case reports
