Protein adsorption onto diethylaminoethyl dextran modi fied anion exchanger:Effect of ionic strength and column behavior☆
Shu Bai,Lingli Gong,Detao Han,Yutong Li,Linling Yu*,Yan Sun
Department of Biochemical Engineering and Key Laboratory of Systems Bioengineering of Ministry of Education,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
1.Introduction
Nowadays,ion exchange chromatography(IEC)has become one of the most competitive tools in industrial large-scale production of pharmaceutical protein[1-3].In particular,owing to the large improvements on both equilibrium adsorption capacity and mass transfer rate than the traditional ungrafted ion-exchangers(surface-ligand resins),functional polymer modified ion-exchangers(grafting-ligand resins and mixedlig and resins)have attracted the attention of researchers worldwide[4,5].The enhanced equilibrium capacity is generally considered due to the three-dimensional binding volume[6].Moreover,the accelerated uptake rate was attributed to surface transport mechanisms(e.g.,activated jump[7,8],electrokinetic effects[9],and“chain delivery”effect[10-12]),shortening protein transfer path[4],and/or other effects in the grafting layer[12,13].Notably,the “chain delivery”effect,which is driven by the chemical potential to ward the bead center as well as the interactions between neighboring flexible polymer chains(and/or between the local polymer chains and the neighboring surface-ligands)mediated by the bound proteins[14],is regarded as the most rational explanation for the fast uptake kinetics in polymer-functionalized ion exchangers.
In our previous work[15],Sepharose FF was modified with diethylaminoethyl dextran(DEAE dextran,DexD)and/or DEAE(D),and three kinds of ion exchangers(surface-ligand resins,FF-D;grafting-ligand resins,FF-DexD;and mixed-ligand resins,FF-D-DexD)with different ionic capacities(ICs)were obtained.Comprehensive analysis on bovine serum albumin(BSA)uptake to these 12 resins disclosed the unique role of the surface-ligands in “chain delivery”effect:as “transfer stations”between two neighboring chains to assist the transport of bound proteins on polymer chains in the mixed-ligand resins.Moreover,all the grafted resins(FF-DexD and FF-D-DexD)afforded excellent binding properties,especially the extremely high uptake rate,proving their superiority in IEC process.
For IEC,which is based on the electrostatic interactions between ionic ligand and charged protein,the ionic strength(IS)has important impacts on protein adsorption and elution behaviors.Generally,protein is bound atlow IS and eluted athigh IS in IEC process.However,the sensitivity to salt concentration is quite different for resins of different ligand chemistries[16],which is decisive in their operating salt concentration range.For instance,it was reported that commercially available dextran-grafted resins Q Sepharose XL(mixed-ligand resin)displayed higher sensitivity on adsorption capacity than the traditional ungrafted ion-exchangers[3],limiting its operating condition at 0-100 mol·L-1NaCl.Whereas the protein adsorption capacities on poly(ethylenimine)(PEI)-modified Sepharose FF resins(grafting-ligand resins)were much less sensitive than the traditional ungrafted resins[17],affording its operating condition up to 200-300 mol·L-1NaCl.Hence,it is worthwhile to explore the effect of IS on adsorption behaviors of the DEAE dextran-grafted resins(both mixed-ligand resins and grafting-ligand resins),for the evaluation of their performances in practical IEC operations.Furthermore,because the “chain delivery”effect greatly depends on the electrostatic interactions between protein molecules and ionic ligands(both the surface-ligand and grafting-ligand)[6]and the salt shields the electrostatic interactions,IS would significantly affect the protein adsorption to DEAE dextran-grafted resins by altering the “chain delivery”effect.
Therefore,in this work,six typical DEAE dextran-grafted resins(three FF-DexD and three FF-D-DexD)were selected.Bath adsorption equilibria and kinetics,linear gradient elution,and breakthrough experiments were performed,using BSA as the model protein,to elucidate the complicated effects of IS(NaCl concentration).Moreover,binary mixture of BSA and γ-globulin was used to evaluate the practical usability of the DEAE dextran-based IEC column.Commercially available resin DEAE Sepharose FF was used for comparison.The results are expected to help optimizing an IEC operation with DEAE dextrangrafted anion exchangers and to bene fit the selection suitable media for protein IEC.
2.Materials and Methods
2.1.Materials
DEAE Sepharose FF was obtained from GE Heal thcare(Uppsala,Sweden).DEAE dextran-grafted resins(FF-DexD50,FF-DexD90,FF-DexD160,FF-D30-DexD50,FF-D100-DexD50,and FF-D60-DexD160)were from our previous work[15].The physical properties of the ion-exchangers are listed in Table 1.BSA(≥96%,MW~ 66400,pI~4.9),γ-globulin(from human blood,≥99%,MW~180000,pI~6.8)were purchased from Sigma-Aldrich(St.Louis,MO,USA).Sodium chloride(NaCl),tris(hydroxy methyl)amino methane(Tris),hydrochloric acid(HCl),and other reagents were of analytical grade from Sangon Biotech Co,Ltd.(Shanghai,China).
Tris-HCl buffers with varying NaCl concentrations(pH 8.0)were prepared and filtered as described previously[9].BSA was dissolved in 20 mmol·L-1Tris-HCl plus NaCl of varying concentrations at pH 8.0 and filtered prior to use through 0.22 μm syringe filters to remove any possible insoluble aggregates and impurities.Protein concentration was determined photometrically with a Lamda 35 UV/VIS spectrophotometer(Shelton,CT,USA)at 280 nm using an extinction coefficient of EmM(280 nm)=45.5 for BSA[18]and E1%(280 nm)=13.8 for γ-globulin[19].
2.2.Batch adsorption isotherms and kinetics
All batch adsorption experiments of BSA on the resins were carried out in 20 mmol·L-1Tris-HCl with varying NaCl concentrations(pH 8.0).The adsorption isotherms were describe by the Langmuir model,

where q is the equilibrium solid-phase concentration of protein,c is the equilibrium liquid-phase concentration,qmis the saturated adsorption capacity,and Kdis the dissociation constant.The qmand Kdvalues with their standard errors are obtained by fitting Eq.(1)to the equilibrium data.
Uptake kinetic experiments were conducted on an ?KTA Explorer?100(GE Heal thcare,Uppsala,Sweden)by the methods described previously with minor modifications[2].Brie fly,after equilibration with the buffer of 20 mmol·L-1Tris-HCl with varying NaCl concentrations(pH 8.0),a certain amount of drained ion-exchanger was added to 100 ml of BSA solution(initial BSA concentration was 1.0 mg·ml-1),prepared in above buffer,in a 200-ml three-neck round-bottom flask,to start the experiment at an agitation speed of 280 r·min-1.By using the ?KTA Explorer? 100 system,the real-time protein concentration in the suspension was monitored at 280 nm with its UV-900 detector,and the solution was circulated by its pump P-950 of through a 2.0 μm stainless steel filter at a flow rate of20 ml·min-1.Ateach kinetic experiment,the mass of each adsorbent was adjusted to achieving a final equilibrium concentration of(0.5 ± 0.1)mg·ml-1.The effective pore diffusivity(De)was determined by fitting experimental uptake data to effective pore diffusion model[20]using the Matlab 7.5.0 software(Math works,USA).It is worth pointing out that Deis just a lumped kinetic parameter describing the overall uptake rate of protein adsorption contributed by complex mechanisms and cannot represent the real kinetic mechanism.For data normalization,the free solution diffusivity(D0)of BSA,6.0 × 10-11m2·s-1at 25 °C[21]was used.Each kinetic experiment was conducted in triplicate and the average value with its standard deviation is reported.
2.3.Chromatographic experiments
All chromatographic experiments were performed on an ?KTA Purifier?10(GE Heal thcare,Sweden)installing a Tricorn? 5/50column(inner diameter 5 mm,length 50 mm,GE Healthcare,Sweden)packed with(1.07± 0.06)ml resins at 25°C.
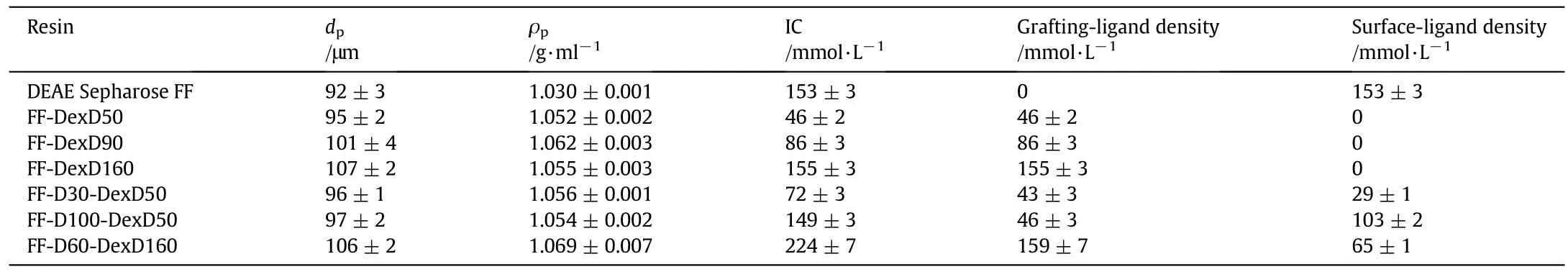
Table 1 Physical properties of ion exchangers①
The breakthrough experiments were performed with the same method described earlier[22].In brief,the column was first equilibrated with fifteen column volumes(CVs)of equilibration buffer(20 mmol·L-1Tris-HCl,IS=0.01 mol·L-1,pH 8)and the A280 was set to zero when the signal became stable.Then,2.0 mg·ml-1of BSA in the equilibration buffer was loaded at a flow rate of 0.5 ml·min-1(150 cm·h-1)until 50%breakthrough was reached.After elution with 1000 mmol·L-1NaCl,the column was regenerated with 0.5 mol·L-1NaOH and equilibrated for the next operation.
The dynamic binding capacity(DBC)at10%breakthrough wascalculated according to the following equation[12,23,24]:

where Vpis the volume of protein solution loaded until 10%breakthrough,Vhthe total holdup volume within the system(the dead volume,from feedstock injection tubing to the UV detector cell),Vbthe packed-bed volume,and Cpthe feed BSA concentration.Each breakthrough experiment was performed in duplicate and it was confirmed that the duplicate breakthrough curves almost overlapped.The average DBC value with its standard deviation is reported.
Linear gradient elution(LGE)chromatography experiments were conducted following the method described by Liu et al.[25]with minor modifications.Brie fly,the column was first equilibrated as described above,and 2.5 ml of BSA(2 mg·ml-1)in the equilibration buffer was loaded(5 mg loading).Thereafter,the column was washed with 4 CVs of the equilibration buffer,and eluted with a linear gradient from IS=0.01 mol·L-1to the finalIS=1.01 mol·L-1in 10 CVs.Finally,the column was regenerated with 0.5 mol·L-1NaOH and equilibrated for the next operation.The mobile phase velocity maintained 0.5 ml·min-1(150 cm·h-1).Salt concentrations at the elution peaks(IRvalues)were obtained from their linear conductivity traces.The recovery of protein(R)was calculated according to the following equation:

where Aeis the peak area in elution and Aris the peak area in regeneration.The amount of each protein flow-through,bound,eluted and regenerated(mg·ml-1bed volume)on all the IEC columns was calculated from their peak areas in corresponding to the total area of 5 mg protein.Each LGE chromatography experiment was conducted in triplicate to ensure reproducibility.The typical chromatogram and the average value with its standard deviation were reported.
Separation of binary protein mixtures was firstly conducted following the method described in LGE experiments with minor modification on loading:2.5 ml of the mixture of BSA(1 mg·ml-1)and γ-globulin(1 mg·ml-1)in the equilibration buffer was loaded(5 mg loading of total protein,and 2.5 mg loading for each protein,respectively),in order to find out the IRvalue of each protein for the step gradient elution.Then,the step gradient elution of the binary protein mixtures were conducted according to the IRvalue for each protein obtained in LGE experiments.Brie fly,2.5 ml of the mixture of BSA(1 mg·ml-1)and γ-globulin(1 mg·ml-1)in the equilibration buffer was loaded.Thereafter,the column was washed with 4 CVs of the equilibration buffer,and eluted with an isocratic step of IS=0.16 mol·L-1with 5.5 CVs,followed by an isocratic elution at IS=0.31 mol·L-1for 4.5 CVs.Finally,the column was regenerated with 0.5 mol·L-1NaOH and equilibrated for the next operation.The mobile phase velocity maintained 0.5 ml·min-1(150 cm·h-1).The recovery of each protein was calculated according to Eq.(3).The contents of elution peaks were analyzed by SDS-PAGE,and the purities of proteins were analyzed by Gel-Pro Analyzer.Each binary separation experiment was conducted in triplicate to ensure reproducibility.The typical chromatogram and the average value with its standard deviation were reported.
3.Results and Discussion
3.1.Adsorption equilibria
Six DEAE dextran-Sepharose FF resins(three FF-DexD and three FFD-DexD resins,respectively)were chosen to determine the effect of IS on BSA adsorption equilibrium and uptake kinetics:FF-DexD160 and FF-D100-DexD50 have similar ICs to the commercial Sepharose-based ungrafted anion exchangers(DEAE Sepharose FF);FF-DexD90 and FF-D30-DexD50 have similar ICs;FF-D60-DexD160 afforded extremely favorable adsorption equilibrium and mass transfer properties(the De/D0value as high as 2.52±0.05[15]);FF-DexD50,FF-D30-DexD50,and FF-D100-DexD50 have similar grafting densities,so as FFDexD160 and FF-D60-DexD160.
Adsorption isotherms of BSA on the six anion exchangers at varying NaCl concentrations are illustrated in Fig.1,and the equilibrium parameters,qmand Kd,with their standard errors fitted by Eq.(1),are listed in Table 2.It can be seen in Fig.1 that all the 6 anion exchangers displayed favorable binding isotherms at each salt concentration,and all the isotherms exhibited a typical behavior of shifting toward lower levels with increasing IS,giving a working/binding IS range of 0-100 mmol·L-1.Moreover,some isotherms in Fig.1 cannot reach a plateau at a very high protein concentration at high NaCl concentration(100-150 mmol·L-1).As known,a fitted qmis not suitable for representing the adsorption ability when the adsorption isotherm do nothave a plateau.Therefore,in order to have a clear view of adsorption ability at different ISs,the adsorbed protein density(qc)at c=2 mg·ml-1,calculated by Eq.(1)using the parameters in Table 2,is used to describe the real adsorption ability.The qcvalues of the six DEAE dextran-grafted resins and results of DEAE Sepharose FF from our previous work[17]are plotted as functions of NaCl concentration in Fig.2.
It is clearly shown in Fig.2 that though all qcvalues decreased with increasing IS,their decreasing magnitudes were different.In detail,for the resins with similar ICs of(152 ± 4)mmol·L-1,the grafted resins FF-DexD160 and FF-D100-DexD50 appeared to have a steeper decrease trend of qcvalues than the traditional ungrafted resin DEAE Sepharose FF in Fig.2.Moreover,FF-D100-DexD50 presented similar qcvalue with DEAE Sepharose FF at100 mmol·L-1NaCl((38 ± 3)mg·ml-1)although the qcvalue of the former was much higher than the latter at 0 mmol·L-1NaCl(197 vs.131 mg·ml-1).The higher capacity sensitivity to NaCl concentration on the DEAE dextran-grafted resins than ungrafted resins at similar ICs were in accordance with the previous reports in commercial dextran grafted ion exchangers[15].The result is probably due to the compaction and/or collapse of grafting layer after adding salt[3,11,17].Because NaCL screened the electrostatic repulsions between the negative-charged DEAE-dextran chains,the DEAE-dextran chain would become less stretchy and less inflated than it at 0 mol·L-1NaCl,and the neighboring chains may intertwine with each other,giving rise to the compaction and/or collapse of grafting layer at high salt[1,3,17,22,25].Those changes in grating chains and layer would lead to the partial shielding of the binding sites and/or the destruction of 3-dimensional binding volume,and finally cause the significant restrained adsorption capacity.Besides,the “high-salt exclusion”of the grafting layer proposed by Bows et al.[3,16,26,27]could also contribute to the result.In the “high-salt exclusion”mechanism,the protein molecule at high salt cannot penetrate the dextran layer as easily as it at low salt,due to the weakened electrostatic attraction between protein and the DEAE-dextran chain at high salt.
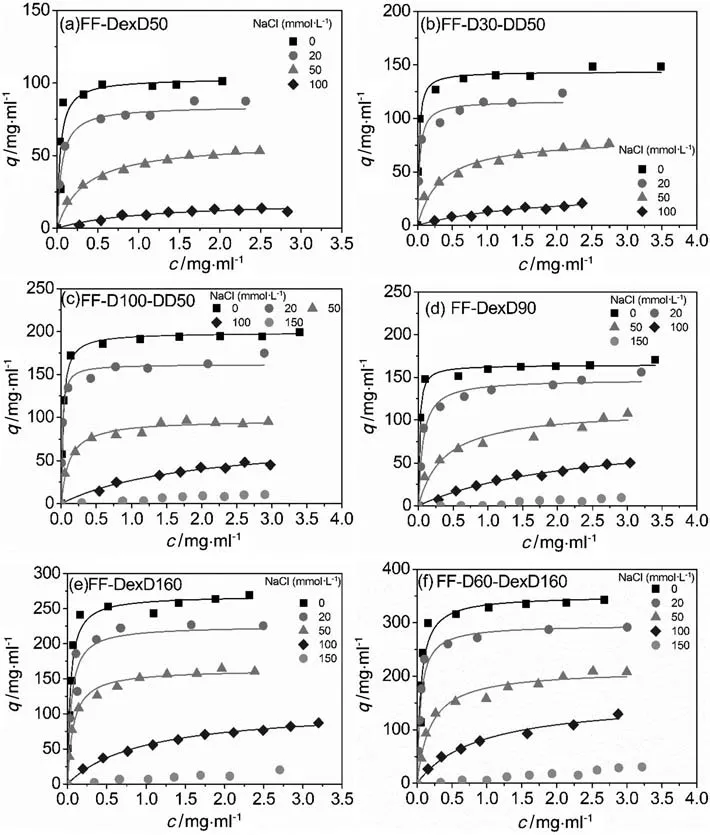
Fig.1.Adsorption isotherms of BSA at different NaCl concentrations in 20 mmol·L-1 Tris-HCl,pH 8.0.(a)FF-DexD50,(b)FF-D30-DexD50,(c)FF-D100-DexD50,(d)FF-DexD90,(e)FFDexD160,and(f)FF-D60-DexD160.The solid lines are calculated from the Langmuir equation.
Besides,the slopes of the curves of FF-DexD50,FF-DexD90,and FF-DexD160 in Fig.2 increased with the increase of grafting-ligand density(89 vs.123 vs.190 mg·ml-1— decrease in qcvalue from 0 to 100 mmol·L-1NaCl),displaying a phenomenon of an increased salt sensitivity with increasing grafting-ligand density.Moreover,steeper decrease trend of qcvalues at higher grafting-ligand density can be seen from the curves of FF-DexD160,FF-D100-DexD50(190 vs.156 mg·ml-1— decrease from 0 to 100 mmol·L-1NaCl)in Fig.2,though they have similar ICs of(152 ± 4)mmol·L-1.The increasedsalt sensitivity with increasing grafting-ligand density is probably because of the severer compaction and/or“salt exclusion”[3,16,26,27]of the thicker grafting layer in this grafted resin.However,qcvalues for the grafting-ligand resin increased with increasing grafting-ligand density atevery NaCl concentration in Fig.2,as well as for the mixed-ligand resins with similar ICs,because of the contribution of 3-dimensional binding volume of the residual grafting layer.
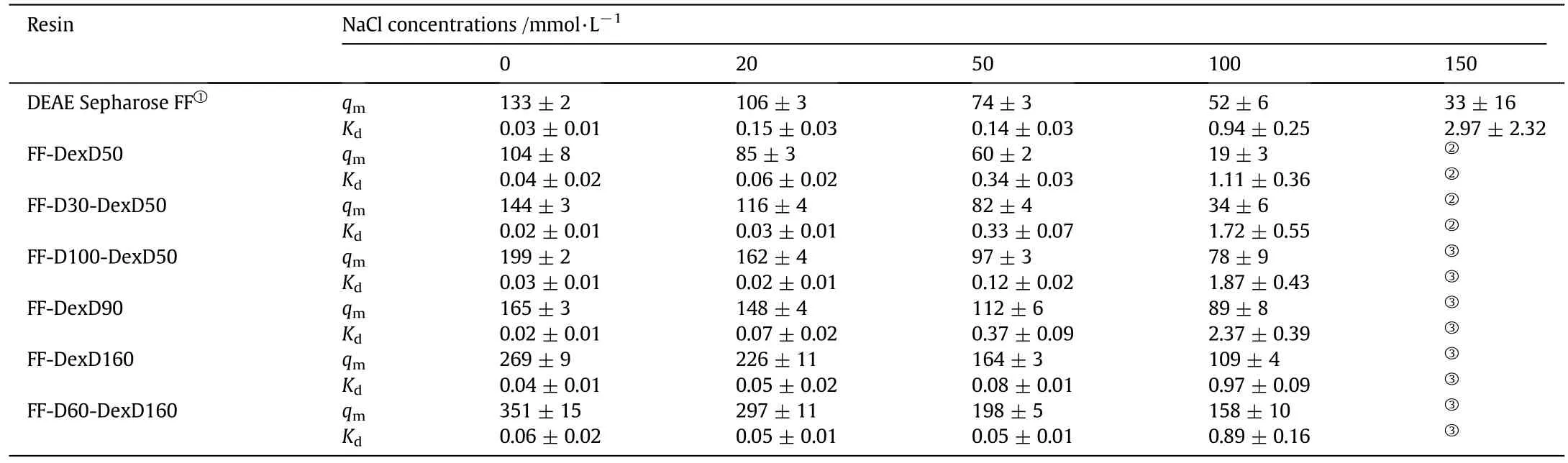
Table 2 Langmuir parameters for BSA adsorption at different NaCl concentrations in 20 mmol·L-1 Tris-HCl,pH 8.0
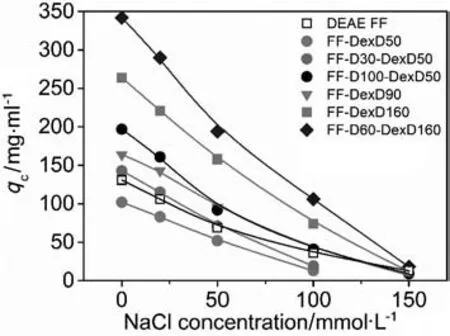
Fig.2.Calculated adsorption density(q c)at an equilibrium liquid-phase concentration of 2 mg·ml-1 as a function of NaCl concentration using the parameters in Table 2.
In addition,for the resins with similar grafting-ligand density of(45 ± 3)mmol·L-1,the slopes of curves of FF-DexD50,FF-D30-DexD50,and FF-D100-DexD50 slightly increased with surface-ligand density in Fig.2(89 vs.124 vs.156 mg·ml-1— decrease in qcvalue from 0 to 100 mmol·L-1NaCl).Similar phenomenon was observed on FF-DexD160 and FF-D60-DexD160(grafting-ligand density of(157 ± 7)mmol·L-1)in Fig.2(190 vs.236 mg·ml-1— decrease in qcvalue from 0 to 100 mmol·L-1NaCl).The higher capacity sensitivity to NaCl concentration at higher surface-ligand density may be attributed to the higher adsorption capacity at 0 mmol·L-1NaCl but similarly little adsorption at high NaCl concentration(Table 2).
Furthermore,it is notable that although DEAE dextran-grafted resins showed higher capacity sensitivity to NaCl concentration,FF-DexD160 and FF-D60-DexD160 afforded high levels of adsorption ability(qc> 70 mg·ml-1)at 0-100 mmol·L-1NaCl.Additionally,they hardly bind protein at 150 mmol·L-1NaCl,suggesting their mild elution condition.
3.2.Uptake kinetics
The kinetic curves of BSA uptake onto the six anion exchangers at different ISs are shown in Fig.3.The pore diffusion model well described the uptake behaviors of all the resins,and the fitted kinetic parameter values,De/D0,with their standard deviations at varying NaCl concentrations were illustrated in Fig.4.
It is found in Fig.4 that with the increase of IS,Devalues of all DEAE dextran-grafted resins increased first and then decreased,while that of the ungrafted DEAE Sepharose FF increased monotonically.The increase-then-decrease trend of Devalues with increasing IS was also reported in PEI-grafted resins[17],and it is mainly attributed to the interplay of two opposite effects of IS on surface diffusion as detailed previously[17].Brie fly,on the one hand,the weakened binding strength(the increased Kdvalues as shown in Table 2)and the increased flexibility of DEAE-dextran chains at higher IS encouraged the happening of“chain delivery”and other forms of surface diffusions.On the other hand,surface diffusion flux(including “chain delivery”)which is directly related to the bound protein density in solid-phase,was limited by the lower adsorption capacity at higher IS(Table 2 and Fig.2).So the maximum of Devalues of the DEAE dextran-grafted resin could be reached at an optimal IS,which was 20 or 50 mmol·L-1NaCl in this work.
Moreover,though the Devalue could be dramatically enhanced by the increase of surface-ligand density for FF-D-DexD resins at 0 mmol·L-1NaCl(Ref.[15]and Fig.4),the enhancement decreased at higher IS for FF-D30-DexD50,and FF-D100-DexD50 compared with FF-DexD50 in Fig.4.The result is considered due to their low density of DEAE-dextran chains.At 0 mol·L-1NaCl,the neighboring chains are far away from each other and some local proteins bound on grafting-ligands cannot be transferred directly by chain swings,so with the assistant of surface-ligand,these local proteins can be transferred through the “transfer station”[15].However,the easier occurrence of “chain delivery”effect at higher IS caused by the weaker binding strength as well as the higher flexibility of DEAE-dextran chains,made the role of the surface-ligand less helpful as “transfer station”,leading to less enhancement on the Devalue.Additionally,after adding NaCl,at the IC values of(152 ± 4)mmol·L-1,the grafting-ligand resin FF-DexD160 afforded higher Devalues than the mixed-ligand resin FF-D100-DexD50 as shown in Fig.4,although the uptake rate of former was slightly faster than the later at0 mol·L-1NaCl(Ref.[15]and Fig.4).The result is considered due to less assistant effect of the surface-ligands in the mixed-ligand resin(weakened facilitating of“transfer station”to chain delivery)as well as the higher contribution of the“chain delivery”as well as the by the higher adsorption capacity in the grafting-ligand resin(Table 2 and Fig.2)after adding salt.These results also suggest the increase of grafting-ligand density in graftinglig and and/or mixed-ligand resin is more effective to achieve high protein uptake performance,than the increase of surface-ligand.
It is remarkable that all 6 DEAE dextran-grafted resins displayed extremely high levels of uptake kinetics(De/D0>1.1)at their working ISs.Especially,FF-DexD160 and FF-D60-DexD160 presented De/D0>1.6 at 0-100 mmol·L-1NaCl,and FF-D60-DexD160 even kept De/D0> 3.0 at 0-50 mmol·L-1NaCl,which is the typical binding IS in protein IEC process.Together with their high adsorption capacity at 0-100 mmol·L-1NaCl and little binding at 150 mmol·L-1as described in Section 3.1,it is proved that FF-DexD160 and FF-D60-DexD160 are excellent candidate for protein IEC operation.
From the above results,it is concluded that though DEAE-dextran grafted resins(both FF-DexD and FF-D-DexD series)were more sensitive to salt concentration than ungrafted DEAE Sepharose FF,they exhibited excellent adsorption equilibrium and kinetic properties at their working ISs,indicating their superiority in IEC.
3.3.Column behavior of BSA
Chromatographic binding behaviors of BSA on the six DEAE-dextran grafted anion-exchangers were characterized by breakthrough experiments(IS=0.01 mol·L-1),and the chromatographic elution behaviors were characterized by LGE experiments(Fig.5).Commercial Sepharose-based ungrafted anion-exchanger DEAE Sepharose FF,was used for comparison.
Their DBC values at10%breakthrough were listed in Table 3.Itis notable that all the six DEAE-dextran grafted anion-exchangers presented higher DBC value(from 147%to 618%)than DEAE Sepharose FF,although the IC of some DEAE-dextran grafted anion-exchangers are lower than the DEAE Sepharose FF,such as FF-DexD50,FF-D30-DexD50,and FF-DexD90.This result is attributed to the much higher Devalue for DEAE-dextran grafted anion-exchangers in Fig.4.It is well-known that,protein DBC was decided by both the adsorption equilibrium and uptake kinetic,and especially by the uptake kinetic[5,16,28].Moreover,whether with the increase of density of graftingligand or surface-ligand,the DBC value increased.This result is in agreement with the accelerated uptake rate with increase ligand density(whatever the grafting-ligand or surface-ligand)reported previously[15].Therefore,it is clearly shown that all the DEAE-dextran grafted resins could provide better binding performance than DEAE Sepharose FF in IEC operation process.
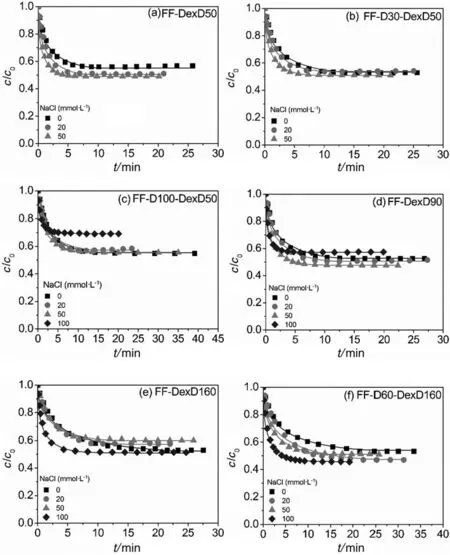
Fig.3.Uptake dynamics of BSA at different NaCl concentrations in 20 mmol·L-1 Tris-HCl,pH 8.0.(a)FF-DexD50,(b)FF-D30-DexD50,(c)FF-D100-DexD50,(d)FF-DexD90,(e)FFDexD160,and(f)FF-D60-DexD160.The solid lines are calculated based on the pore diffusion model with parameters in Table 2 and Fig.4.
Besides,as shown in Fig.5,a single narrow symmetrical elution peak was observed on each of the IEC resins,and elution peaks almost overlapped for them,indicating their similar and favorable elution behaviors.Moreover,there are only tiny regeneration peaks thatexisted in Fig.5,showing a negligible amount of BSA in regeneration.For a better view of the chromatographic elution behavior,salt concentrations at the elution peaks(IRvalues)acquired from their linear conductivity traces,as well as the recoveries of BSA on ion-exchange columns are given in Table 3.It is obvious their IRvalues are quite similar((210±10)mmol·L-1),as wellas theirexcellentrecoveries(97%±2%).The result also suggests mild elution conditions(IR≤ 0.23 mol·L-1)of BSA were promising for all the DEAE-dextran grafted resins,similar with or even milder than the commercial DEAE Sepharose FF,to achieve the similar or even more favorable recoveries.
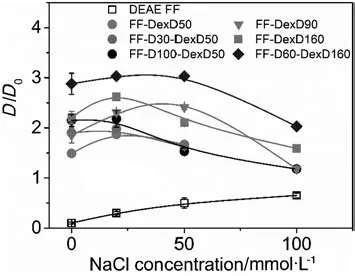
Fig.4.Effective pore diffusivities of BSA on different anion-exchangers as a function of NaCl concentration.The date of DEAE Sepharose FF are from Ref.[17].
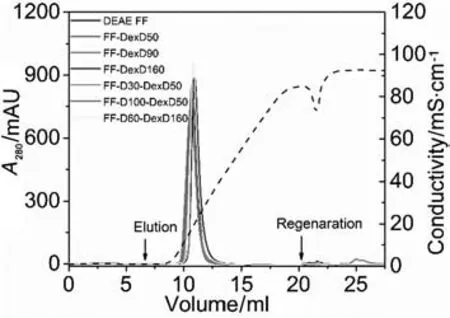
Fig.5.Linear gradient elution chromatograms of BSA from different anion-exchange chromatographic columns.Loading buffers were 20 mmol·L-1 Tris-HCl(pH 8).Gradient duration was 10 CVs from the loading buffer to 20 mmol·L-1 Tris-HCl plus 1000 mmol·L-1 NaCl(pH 8).Flow rate was 0.5 ml·min-1 and loading amounts was 5 mg.The solid lines represent UV signals and the dashed line represents conductivity.
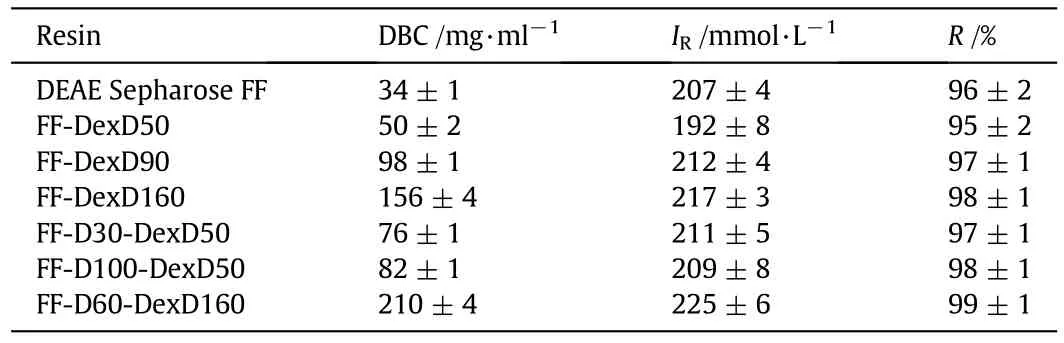
Table 3 Dynamic binding capacities(DBC),salt concentrations at the elution peaks(I R)and recoveries(R)of ion exchangers
Allthe above findings demonstrate that DEAE-dextran grafted resins(both FF-DexD and FF-D-DexD)exhibited favorable adsorption equilibrium,uptake kinetic,column dynamic binding,and chromatographic elution properties in IEC operation conditions,and the DEAE-dextran grafted resin is a series of protein ion-exchangers with high chromatographic performance.
3.4.Separation of binary protein mixtures
It is widely known that γ-globulin is a valuable therapeutic protein existed in serum,so the separation of γ-globulin from protein mixtures is very important.Considering that serum albumin is the main content of the proteins in serum,BSA and γ-globulin were chosen as the two model proteins for the binary mixtures.It is obvious that FF-D60-DexD160 affords the most excellent performance on protein IEC in those DEAE-dextran grafted resins based on above results,thus it was chosen for the investigation of the separation of the binary protein mixtures for the full evaluation of the practical application potentials of the DEAE dextran-based IEC column,with the comparison of commercial ungrafted DEAE Sepharose FF.
Chromatographic binding and elution behaviors of BSA on the anion-exchangers were firstly characterized by LGE experiments,and the chromatograms are provided in Fig.6.It is obvious that similar LGE results were obtained for the two IEC resins.Thatis,though the two proteins cannot be separated well by this linear gradientof 10 CVs from 0 mmol·L-1NaCl to 1000 mmol·L-1NaCl,distinctly different IRvalues for the two protein can be obtained,which are given in Table 4.It is obvious in Table 4 that BSA kept the same IRvalue((208 ± 4)mmol·L-1for DEAE Sepharose FF and(226 ± 3)mmol·L-1for FF-D60-DexD160,respectively)in the binary protein mixtures as that in the single protein solution in Section 3.3(Fig.5 and Table 3).For γ-globulin,due to its much higher molecular weight and lower net charge at pH 8 than BSA[29],its IRvalue were lower than BSA((72 ± 6)mmol·L-1lower for DEAE Sepharose FF and(121 ± 3)mmol·L-1lower for FF-D60-DexD160,respectively).Besides,because of the much smaller pore size of FF-D60-DexD160 than DEAE Sepharose FF[15],γ-globulin as a protein of large size,only could enter limited pore volume of FF-D60-DexD160,giving rise to the lower binding amount(higher area of flow-through peak)and faster elution(lower IRvalue(106±2)mmol·L-1vs.(136 ± 6)mmol·L-1)of FF-D60-DexD160.These results were consistent with the previously reported large protein binding and elution on dextran grafted ion exchangers[1,3,16,26,30].Furthermore,there is little regeneration peak area in Fig.5,showing the high effectivity of elution.The result suggests that using the IRvalue of each protein as the isocratic elution condition would be an effective separation strategy of the binary protein mixtures.
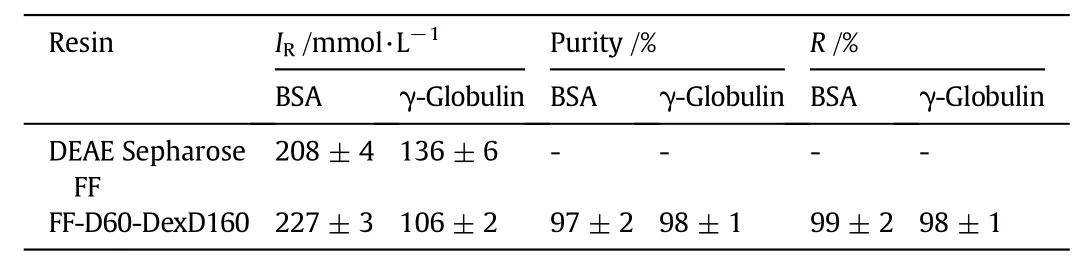
Table 4 Saltconcentrations atthe elution peaks(I R)ofDEAESepharose FF and FF-D60-DexD160 in separation of binary protein mixtures with LGE,and the purity as well as the recovery of proteins obtained from FF-D60-DexD160 by step gradient elution
Hence,the step gradient elution of the binary protein mixtures was conducted on FF-D60-DexD160,and the NaClconcentrations for protein elution were selected according to their IRvalues obtained in the LGE experiment(a little higher than each IRvalue in order to obtain the quick elution).The chromatogram and its SDS-PAGE analysis are provided in Fig.7.Itis clear thatthe two elution peaks with high resolutions were presented in Fig.7(a).Additionally,as showing Fig.7(b),in the elution fractions,high purities were achieved for both of two proteins,indicating the high efficiency ofthe step gradientelution.In order to obtain a more clear view of the chromatographic elution fraction,the purities of the two proteins obtained by the Gel-Pro Analyzer are given in Table 4,and both of them presented high values of>97%.Besides,the tiny regeneration peak in Fig.7 also gave high recoveries ofthe two proteins,as listed in Table 4.The above results proved the two proteins in the binary mixtures were well separated by an isocratic elution of 150 mmol·L-1NaCl of 5.5 CVs followed by an isocratic elution of 300 mmol·L-1NaCl of 4.5 CVs.
Therefore,all the points discussed above demonstrate that DEAE-dextran grafted resins(both FF-DexD and FF-D-DexD),as a novel series of protein IEC resins,not only afforded favorable adsorption and chromatography performance for the single protein,but also exhibited excellent separation efficiency for the protein mixtures,indicating their practical usefulness in protein IEC separation and purification.
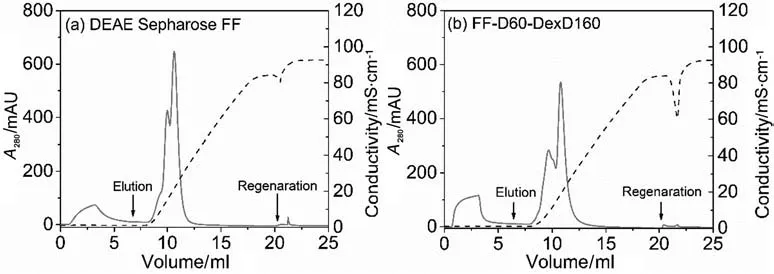
Fig.6.Linear gradientelution chromatograms ofbinary mixtures ofBSA andγ-globulin from differentanion-exchanger chromatographic columns.(a)DEAE Sepharose FF and(b)FF-D60-DexD160.Loading buffers were 20 mmol·L-1 Tris-HCl(pH 8).Gradientduration was 10 CVs from the loading bufferto 20 mmol·L-1 Tris-HClplus 1000 mmol·L-1 NaCl(pH 8).Flow rate was 0.5 ml·min-1 and loading amounts was 2.5 mg BSA and 2.5 mg γ-globulin.The solid lines represent UV signals and the dashed lines represent conductivities.
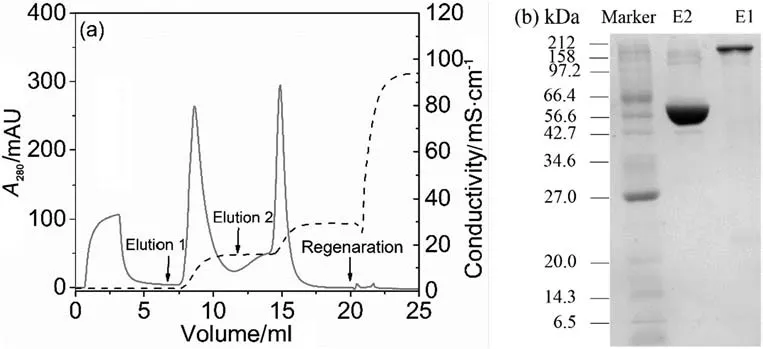
Fig.7.(a).Step gradientelution chromatogram of 2.5 ml loading of binary protein mixtures of2.5 mg BSA and 2.5 mg γ-globulin from FF-D60-DexD160.(b)SDS-PAGE analysis ofthe two elution fractions(E1 for elution 1 of 8-11 ml and E2 for elution 2 of 14-16 ml).Loading buffers were 20 mmol·L-1 Tris-HCl(pH 8).Isocratic elution 1 was 20 mmol·L-1 Tris-HCl plus 150 mmol·L-1 NaCl(pH 8)of5.5 CVs,and isocratic elution 2 was 20 mmol·L-1 Tris-HClplus 300 mmol·L-1 NaCl(pH 8)of4.5 CVs.Flow rate was 0.5 ml·min-1.The solid line represents UV signal and the dashed line represents conductivity.
4.Conclusions
In this work,we have focused on the effect of IS on protein adsorption to six DEAE-dextran grafted ion-exchangers(FF-DexD and FF-DDexDseries).Protein adsorption capacities on the DEAE-dextran grafted resins were more sensitive to NaCl concentration than the commercial ungrafted resin,consistent with the dextran-grafted ion-exchangers reported in literature.Additionally,steeper decrease of adsorption capacities could be observed with increasing either grafting-ligand or surface-ligand density.Besides,the Devalues of those DEAE dextran-FF resins increased first and then decreased with increasing IS,and kept extremely high levels(De/D0>1.1)at their working IS range.It is notable that the De/D0value of FF-D60-DexD160 even kept>3.0 at 0-50 mmol·L-1NaCl,indicating its advantages as an ion-exchanger.It is worth pointing out that the helpful role of surface-ligand to the“chain delivery”effect was weakened after adding salt,so the resins with high grafting-ligand density are more favorable for protein uptake.Furthermore,chromatographic column binding and elution results demonstrate that all the DEAE dextran-FF resin afforded higher DBC value and similar(or even milder)elution condition to achieve the similar(or even higher)recovery,with comparison to DEAE Sepharose FF.Finally,good separation of binary mixtures of BSA and γ-globulin could be easily achieved with both high recovery and purity by step gradient elution,using FF-D60-DexD160 column.These findings have proved the superiority of the series of DEAE-dextran grafted resins in protein IEC,bene fiting the selection of suitable IEC adsorbents and optimization of practical IEC processes.
Considering the exchange of counterions and protein molecules is crucial to protein adsorption and elution,and the IEC performance of polymer-grafted ion-exchangers is significantly in fluenced by the counterions[22],our next work will be directed toward the in fluence of counterions on protein adsorption equilibria,uptake kinetics,DBC,and column elution of typical DEAE-dextran grafted resins.
Nomenclature
Aepeak area in elution
Arpeak area in regeneration
Cpfeed BSA concentration,mg·ml-1
c free protein concentration in equilibrium,mg·ml-1
Deeffective pore diffusivity of the protein,m2·s-1
D0free solution diffusivity of hIgG(4.0 × 10-11m2·s-1at25 °C)
dpvolume-weighted average diameter of a resin,μm
IRsalt concentration at each elution peak,mg·ml-1
Kddissociation constant,mg·ml-1(or mmol·L-1)
q adsorption density of protein,mg·ml-1
qcadsorbed protein density at an equilibrium liquid-phase concentration,mg·ml-1
qmadsorption capacity of protein,mg·ml-1
R recovery of protein,%
Vbpacked-bed volume,ml
Vhtotal holdup volume,ml
Vpvolume ofprotein solution loaded until 10%breakthrough,ml
ρpdensity of the hydrated particles,mg·ml-1
[1]A.M.Lenhoff,Protein adsorption and transport in polymer-functionalized ionexchangers,J.Chromatogr.A 1218(49)(2011)8748-8759.
[2]L.L.Yu,Q.H.Shi,Y.Sun,Effect of dextran layer on protein uptake to dextran-grafted adsorbents for ion-exchange and mixed-mode chromatography,J.Sep.Sci.34(21)(2011)2950-2959.
[3]B.D.Bowes,H.Koku,K.J.Czymmek,A.M.Lenhoff,Protein adsorption and transport in dextran-modi fied ion-exchange media.I:adsorption,J.Chromatogr.A 1216(45)(2009)7774-7784.
[4]E.Müller,Comparison between mass transfer properties of weak-anion-exchange resins with graft-functionalized polymer layers and traditional ungrafted resins,J.Chromatogr.A 1006(1-2)(2003)229-240.
[5]Q.H.Shi,G.D.Jia,Y.Sun,Dextran-grafted cation exchanger based on superporous agarose gel:adsorption isotherms,uptake kinetics and dynamic protein adsorption performance,J.Chromatogr.A 1217(31)(2010)5084-5091.
[6]L.L.Yu,S.P.Tao,X.Y.Dong,Y.Sun,Protein adsorption to poly(ethylenimine)-modi fied Sepharose FF:I.A critical ionic capacity for drastically enhanced capacity and uptake kinetics,J.Chromatogr.A 1305(2013)76-84.
[7]K.Miyabe,G.Guiochon,Restricted diffusion model for surface diffusion in reversedphase liquid chromatography,Anal.Chem.72(7)(2000)1475-1489.
[8]K.M.And,S.Takeuchi,Analysis of surface diffusion phenomena in liquid phase adsorption,J.Phys.Chem.B 101(39)(1997)7773-7779.
[9]L.Yu,L.Zhang,Y.Sun,Protein behavior at surfaces:orientation,conformational transitions and transport,J.Chromatogr.A 1382(2015)118.
[10]Y.Tao,G.Carta,G.Ferreira,D.Robbins,Adsorption of deamidated antibody variants on macroporous and dextran-grafted cation exchangers:I.Adsorption equilibrium,J.Chromatogr.A 1218(11)(2011)1519-1529.
[11]M.C.Stone,G.Carta,Protein adsorption and transport in agarose and dextrangrafted agarose media for ion exchange chromatography,J.Chromatogr.A 1146(2)(2007)202-215.
[12]J.Th?mmes,Investigations on protein adsorption to agarose-dextran composite media,Biotechnol.Bioeng.62(3)(1999)358.
[13]E.Müller,Properties and characterization of high capacity resins for biochromatography,Chem.Eng.Technol.28(11)(2010)1295-1305.
[14]A.Staby,J.H.Jacobsen,R.G.Hansen,U.K.Bruus,I.H.Jensen,Comparison of chromatographic ion-exchange resins V.Strong and weak cation-exchange resins,J.Chromatogr.A 1118(2)(2006)168-179.
[15]L.Yu,L.Gong,S.Bai,Y.Sun,Surface DEAE groups facilitate protein transport on polymer chains in DEAE-modi fied-and-DEAE-dextran-grafted resins,AIChE J.62(10)(2016)3812-3819.
[16]B.D.Bowes,A.M.Lenhoff,Protein adsorption and transport in dextran-modi fied ionexchange media.II.Intraparticle uptake and column breakthrough,J.Chromatogr.A 1218(29)(2011)4698.
[17]L.L.Yu,Y.Sun,Protein adsorption to poly(ethylenimine)-modi fied Sepharose FF:II.Effect of ionic strength,J.Chromatogr.A 1305(2013)85-93.
[18]Y.J.Lee,A.C.Notides,Y.G.Tsay,A.S.Kende,Coumestrol,NBD-norhexestrol,and dansyl-norhexestrol, fluorescent probes of estrogen-binding proteins,Biochemistry 16(13)(1977)2896.
[19]M.T.Tyn,T.W.Gusek,Prediction of diffusion coefficients of proteins,Biotechnol.Bioeng.35(1990)12.
[20]H.Pedersen,L.Furler,K.Venkatasubramanian,J.Prenosil,E.Stuker,Enzyme adsorption in porous supports:local thermodynamic equilibrium model,Biotechnol.Bioeng.27(7)(2010)961-971.
[21]L.He,B.Niemeyer,A novel correlation for protein diffusion coefficients based on molecular weight and radius of gyration,Biotechnol.Prog.19(2)(2003)544.
[22]N.Liu,L.Yu,Y.Sun,Protein adsorption to poly(ethylenimine)-modi fied Sepharose FF:V.Complicated effects of counterions,J.Chromatogr.A 1404(2015)44-50.
[23]G.Carta,A.Jungbauer,Protein Chromatography:Process Development and Scale-Up,Wiley,John&Sons,2010.
[24]A.M.Hardin,C.Harinarayan,G.Malmquist,A.Axén,R.V.Reis,Ion exchange chromatography of monoclonal antibodies:effect of resin ligand density on dynamic binding capacity,J.Chromatogr.A 1216(20)(2009)4366-4371.
[25]N.Liu,L.L.Yu,Y.Sun,Protein adsorption to poly(ethylenimine)-modi fied Sepharose FF.IV.Dynamic adsorption and elution behaviors,J.Chromatogr.A 1362(2014)218-224.
[26]B.D.Bowes,A.M.Lenhoff,Protein adsorption and transport in dextran-modi fied ionexchange media.III.Effects of resin charge density and dextran content on adsorption and intraparticle uptake,J.Chromatogr.A 1218(40)(2011)7180-7188.
[27]B.D.Bowes,S.J.Traylor,S.M.Timmick,K.J.Czymmek,A.M.Lenhoff,Insights into protein sorption and desorption on dextran-modi fied ion-exchange media,Chem.Eng.Technol.35(1)(2012)91-101.
[28]Q.H.Shi,X.Zhou,Y.Sun,A novel superporous agarose medium for high-speed protein chromatography,Biotechnol.Bioeng.92(5)(2005)643.
[29]Y.Hong,N.Liu,W.Wei,L.L.Yu,G.Ma,Y.Sun,Protein adsorption to poly(ethylenimine)-modi fied Sepharose FF:III.Comparison between different proteins,J.Chromatogr.A 1342(2014)30-36.
[30]L.L.Gong,B.Shu,L.L.Yu,Studies on synthesis and application of novel diethylaminoethyl dextran modi fied anion exchanger for antibody puri fication,Ion Exch.Adsorpt.32(6)(2016)501-510.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Surface chemical characterization of deactivated low-level mercury catalysts for acetylene hydrochlorination☆
- Insight into fouling behavior of poly(vinylidene fluoride)(PVDF)hollow fiber membranes caused by dextran with different pore
- Gas emission source term estimation with 1-step nonlinear partial swarm optimization-Tikhonov regularization hybrid method☆
- Dissolution of antibiotics mycelium in ionic liquids:Performance and mechanism☆
- Kinetic studies on extra heavy crude oilupgrading using nanocatalysts by applying CFD techniques☆
- Effect of the operation parameters on the Fischer-Tropsch synthesis in fluidized bed reactors☆
