Effects of Winter Cover Crop and Straw Returning on the Functional Diversity of Rhizosphere Microf lora in Double-crop Rice Paddies
TANG Hai-ming, XIAO Xiao-ping, LI Chao, TANG Wen-guang, GUO Li-jun, WANG Ke, SUN Yu-tao, CHENG Kai-kai, SUN Geng, PAN Xiao-chen
Hunan Soil and Fertilizer Research Institute, Changsha 410125, PRC
Abstract The functional diversity of rhizosphere microf lora which is also known as the “microbial community” is a sensitive indicator of soil quality subject to the type of winter cover crop and straw returning. In order to evaluate the effects of different winter cover crops and returning patterns on the functional diversity of rhizosphere microflora in double-crop rice paddies, we designed f ive winter cover crops and straw returning combinations to analyze their effects on the functional diversity of rhizosphere microflora in rice paddies: ryegrass (Lolium multiflorum L.)-double-crop rice (Ry), milk vetch (Astragalus sinicus L.)-double-crop rice (Mv), Rape (Brassica napus L.)-Double-crop rice (Ra), Potato (Solanum tuberosum L.)-double-crop rice (Po), and winter fallow-doublecrop rice (CK, the control). In this paper, the average well color development (AWCD) in Biolog-GN plates indicated the capacity for carbon utilization by the rhizosphere microbial community. We analyzed the rhizosphere microbial community functional diversity of the paddy soils with the above f ive treatments by using the Biolog-GN system. The results showed that applications of winter cover crop and straw returning caused high increases in AWCD compared with CK, and the AWCD values for samples with Po treatment was greater than those with Ry and CK treatments at the early and late rice maturity stages. It was concluded that applications of winter cover crop and straw returning can cause changes in the carbon utilization eff iciency of rhizosphere microf lora. There were differences in the genetic diversity of the rhizosphere microf lora among different treatments at the maturity stage of early and late rice. The richness, Shannon, and McIntosh Index under different winter cover crop and straw returning treatments were signif icantly different. The highest indexes were observed in the Po treatment and the lowest in the CK at the maturity stage of early and late rice. The richness, Shannon, and McIntosh Index under different treatments ranked in descending order is as follows: Po>Ra>Mv>Ry>CK. Principal Component Analysis (PCA) of substrate reactions were conducted in this research. The results indicated that the pattern of carbon source utilization varied with winter cover crop treatments, and that carbohydrates and amino acids were the main carbon sources of rhizosphere microorganisms. To conclude, the application of winter cover crop and straw returning to paddy fields could signif icantly increase the carbon source utilization, species richness, and species evenness of rhizosphere microf lora in double-crop rice paddies.
Key words Rice; Winter cover crop; Double-croppaddies; Rhizosphere soil; Functional diversity of microf lora
1. Introduction
Microorganisms, a vital soil component, could provide useful information about soil quality and therefore are widely used to measure the physicoch- emical properties of soil, the ecological environment, and the growth of plants[1]. Changes in the functional diversity of microf lora are precursors to changes in the ecological properties of the microorganisms in the soil. As a well-known method for testing the functional and structural diversity of soil microorganisms[2], Biolog EcoPlate Analysis reflects the physiological outline of the microbial community by examining the carbon source utilization of microorganisms[3]. Basically, Biolog-GN plates can identify all species of carbon sources[4]. Biolog is very popular in the evaluation of the ecological environment and in microbial detection in recent years[5], mainly because it’s easy to operate and can provide accurate information about the activity of the microbial community.
The functional diversity of microf lora is subject to the change in the ecological environment of rice paddies. Scholars have carried out extensive researches to investigate the factors influencing the structural and functional diversity of microflora, such as the growing pattern, soil utilization, soil type, soil physiochemical properties, fertilization, straw mulching, soil tillage, water management, etc. BUENEMANN et al.[6]proved that the type of crops, and their growing patterns have signif icant effects on the structure and diversity of the microbial community. ZHANG J et al.[7]investigated the functional diversity of microf lora under different soil utilization patterns. They found that the returning of farmland to lake is conducive to the recovery of the structure of the microbial community in wetland regions. SHI P et al.[8]reported that continuous cultivation of corn combining straw mulching and fallow could improve the metabolic activity of microorganisms as well as the functional diversity of the soil microflora. GARLAND et al.[2]suggested that the structure of the microbial community varies greatly with the type of soil. HU et al.[9]reported that long-term and balanced fertilization can enhance the functional activity of microorganisms and the contents of microbial biomass such as carbon and nitrogen. HOU X J et al.[10]believed that proper fertilization could help strengthen the average well color development (AWCD) and effectively improve microbial diversity. XU Y L et al.[11], LUO X Q et al.[12], and XU W L et al.[13]reported that the application of organic and inorganic fertilizers can both improve the microbial diversity in soils. ZHOU W X et al.[14]observed a significant increase in the functional diversity of soil microbes under treatments with 67% and 100% rice straw mulching; the increase peaked at 67% rice straw mulching. Whereas there were also research findings indicating declines of microbial activity caused by the long-term application of inorganic nitrogenous fertilizers[15].
Hunan Province, the main producer of doublecrop rice, held great strategic signif icance for national food security. The preservation and maintenance of soil quality is the key to securing the high yield and stable harvest of rice grains. The planting of crops in winter is an important part of sustainable agriculture. It is reported that the total area of fallow paddies in South China has reached 2.00×107hm2, among which 5.89×106hm2was used for growing rape (Brassica napus), 1.86×106hm2for milk vetch (Astragalus sinicus), 1.22×106hm2for potatoes (Solanum tuberosum), and 7.67×105hm2for ryegrass (Lolium multiflorum)[16]. Southern rice production regions have superior natural conditions involving lighting, temperature, water and soil texture. The development of winter agriculture not just improves the utilization of fallow paddies, it also enhances the coverage of paddies in winter by green crops. The growing of winter crops in fallow paddies can prevent soil erosion, increase carbon-nitrogen contents, and improve physiochemical properties of the soil. It has been proved to be an effective method to enhance the grain yield of later-crops and maintain sustainable development[17-18]. Extensive researches have been done to investigate the relationship between winter cover crop and straw returning in double-crop rice paddies from various aspects, such as the nutrients and physiochemical properties of paddy soils, greenhouse gas emission, the yield of rice grain, and the physiochemical properties of later-crop rice[19-23]. However, there was little amount of research concer- ning the inf luence of winter crop mulching and straw application on the functional diversity of rhizosphere microflora in fallow paddies of double-crop rice. In this paper, 4 typical winter cover crops of southern rice production regions including ryegrass, milk vetch, rape and potato were chosen as the materials to examine their inf luence on the functional diversity of rhizosp- here microf lora using Biolong-GN technology. Taken together, the experiment included 5 growing patterns: ryegrass-double-crop rice (Ry), milk vetch-doublecrop rice (Mv), rape-double-crop rice (Ra), potatodouble-crop rice (Po), and winter fallow-doublecrop rice (CK, the control). The f indings of this paper can provide a useful theoretical support for the deve- lopment of winter cover crop-double-crop rice patterns.
2. Materials and Methods
2.1. Experiment site
The experiment was launched in October 2014 in the purple alluvial paddies of Sanfengsi, Huarong, Hunan. Basic nutrients of the surface soil before the experiment included organic matter (30.96 g/kg), total nitrogen (2.01 g/kg), total phosphorus (0.48 g/kg), total potassium (17.70 g/kg), available nitrogen (166.60 mg/kg), available phosphorus (10.04 mg/kg), rapidly available potassium (72.00 mg/kg), and a pH value of 6.20. The humid continental monsoon climate provided an average annual temperature of 17.0~18.0 ℃ and an average annual precipitation of 1 200 mm; the active accumulated temperature ≥10℃ was 5 000~5 600℃; the duration of the frost-free season reached 260 d.
2.2. Experiment design and field management
The experiments were conducted under 5 treatments: ryegrass-double-crop rice (Ry), milk vetch-double-crop rice (Mv), rape-double-crop rice (Ra), potato-double-crop rice (Po), and winter fallowdouble-crop rice (CK). Each treatment included three replications. Blocks were arranged randomly; each block was 50.0 m2in area and 20.0 m (length)×2.5 m (width) in dimension. The ridges of the blocks were wrapped with plastic mulch to ensure independent nutrient and water management. Detailed field management and fertilization measures are listed in Table 1. The water management of early and late rice during growth periods included shallow irrigation at the early stage, drying paddy field in sunshine at the middle stage, and alternation of drying and wetting at the late stage. Other management measures were the same as regular field production, such as conventional irrigation, fertilization, and pestcontrol.
2.3. Collection and determination of samples
When the rice entered the maturity stage, rhiz- osphere soils were collected (early rice on July 15th, 2017 and late rice on October 17th, 2017) from multiple sites of a block and evenly mixed to form a sample of the block. There were three replications for each block. Detailed sampling steps: dig out the rice roots from the sunshine-dried paddies; gently shake off the soil body loosely attached to the roots; brush off the soil closely attached to the root in the range of 0~4 mm as rhizosphere soils[24]; remove the impurities (e.g. stones and straw stems), rapidly pour the samples into plastic bags, put the bags in ice boxes, and store them in the laboratory refrigerator at 4℃. Biolog-GN was conducted 48 h after the sampling. Meanwhile, fresh soil samples were collected from the paddy at a depth of 0~4 cm in the maturity stage of late rice under each treatment. These samples were dried in the air and sieved before the test of physiochemical properties, including soil pH value (soil ∶water=1 ∶2.5) and the contents of organic matter (potassium dichromate method), total nitrogen (potassium dichromatesulfuric acid digestion), available nitrogen (alkaline hydrolysis diffusion), available phosphorus (NaHCO3extraction-Mo-Sbcolorimetry), and rapidly available potassium (NH4Acextraction-f lame photometry)[25].
A 96-well microplate was used in the Biolog-GN test. Each well contained a certain type of carbon source and some redox dye (nitroblue-tetrazolium), with the exception of the first well for carbon-free control. The changes in redox potential caused by the respiration of microorganisms via C source would lead to a color change of tetrazolium compounds (TV) from colorless to purple[26]. The light absorption value and its variance of each well were measured to ref lect the metabolic diversity of the microbial community. Detailed procedures of the test were: fresh soil sample was pre-incubated at 26℃ for 4~6 h. 95 mL of sterile NaCl solution (0.145 mol/L) was added to 10 g of the pre-incubated soil. The mixture was treated in the shaker for 15 min (5 g soil+95 mL sterile water). The mixture was diluted with sterile water to 10-3. 125 μL of suspension was inoculated to each well on the microplate. The microplate was incubated at 25℃. The plate was read every 12 h with a Biolog reader (wavelength: 590 nm). The test lasted for 12 d[27].

Table 1 Field management of winter crop and double-crop rice in growth period
2.4. Data processing
The average well color development (AWCD) was calculated with Formula (1). The number of micro wells (OD590>0.15) was used as the Richness Index of the sample soil to evaluate the diversity of the species[28]. The Shannon Index (H) was calculated by Formula (2) to evaluate the richness of the species, and the Mclntosh Index (U) was calculated by Formula (3) to assess the evenness of the species[2,29].


C is the light absorption value of the carbon well. R is the light absorption value of the control well. n is the number of wells containing carbon source. For the GN microplate, n=95. Pi is the ratio of the i-th well’s relative light absorption value to the total relative light absorption values of all reaction wells, namely, Pi= (C-R)/∑(C-R).
Experiment data were processed with Excel 2003. Variance analysis, principal components analysis, and multiple comparisons were completed with DPS 3.11 (Data Processing System for Practical Statistics). Multiple comparisons were based on the LSD method (P<0.05). CCA analysis was conducted using the CANOCO software package.
3. Results and Analysis
3.1. AWCD of microplate wells
Average well color development (AWCD) is a critical indicator of single carbon source utilization by the microbial community in soils. It is widely applied to determine the microbial activity of soil microorganisms as well as their functional diversity[2]. The AWCD of the rhizosphere soil tended to vary with the type of rice involving early rice and late rice in the maturity stage and presented a trend of increasing (Fig. 1). Specif ically, the rapid growing of the AWCD with the incubation time slowed down at 108 h.
The incubation lasted for 276 h, presenting significant variance in the AWCD with the type of winter cover crop (Fig. 1). In the maturity stage of early rice, the AWCDs of winter cover crop treatments were higher than that of CK. The AWCD peaked in the Po treatment, it was higher than that in Ry, Ra and CK (P<0.05). The AWCD in Mv ranked second, much higher than Ra and CK (P<0.05). In the maturity stage of late rice, the AWCDs of Po, Ra, and Mv were much higher than that of CK (P<0.05). The maximal AWCD appeared in Po treatment, it was much higher than Ry, Mv and CK (P<0.05). The AWCD of Ra came second, it was also much higher than Ry and CK (P<0.05). The AWCD of Mv was higher than that of Ry but without signif icant difference (P>0.05).
3.2. Metabolic diversity of the microf lora in the soil
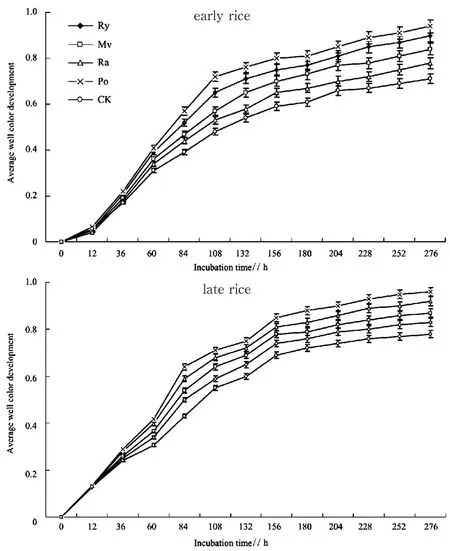
Fig. 1 AWCD changes with incubation time in the maturity stage of early rice and late rice under different treatments(Ry: ryegrass-double-crop rice; Mv: milk vetch-double-crop rice; Ra: rape-double-crop rice; Po: potato-double-crop rice; CK: winter fallow-doublecrop rice. Results were means ± standard errors.)
The Richness Index, Shannon Index, and Mclntosh Index in the rice maturity stage are indicators of microbial richness, diversity and evenness in the soil, which showed significant improvements under various winter cover crop treatments (Table 2). During the maturity stage of early rice and late rice, the Richness and Shannon Index of Po were much higher than those of CK (P<0.05), ranked in descending order as Po>Ry>Mv>Ra>CK and Po>Ra>Mv>Ry>CK, respectively. The Mclntosh Index reached its peak in the Po treatment. It means that the returning of the potato produced the highest evenness of rhizosphere microorganisms. The Mclntosh Index of the Mv treatment came second. The minimal value was observed in CK. Differences existed between the evenness and diversity of the microorganisms in the rhizosphere soil under different treatments.
3.3. Principal components of the microflora’s metabolic diversity
According to the requirements of previous research, the cumulative variance contribution rate of the extracted components should reach 85%[30]. All together, 8 components were extracted in this paper, presenting a cumulative contribution rate of 86.22% and 87.35%. The variance contribution rates of the 1stand 2ndprincipal components (namely, PC1 and PC2) of early rice in the maturity stage were 33.26% and 16.57% respectively. The contribution rates of the 3rdto 8thprincipal components decreased sharply to 8.85%, 7.75%, 6.28%, 5.38%, 4.28%, and 3.85%, respectively. In the maturity stage of late rice, the variance contribution rates of PC1 and PC2 were 35.57% and 17.58% respectively. The contribution rates of the 3rdto 8thprincipal components declined sharply to 8.63%, 7.15%, 6.26%, 4.57%, 4.03%, and 3.56% respectively. Hence, only the f irst two principal components were analyzed (Fig. 2).
Significant differences in the PC axis can be observed under different treatments. The contribution rates scattered around the PC1 axis: the CK treatment distributed mainly along the negative direction. The Po, Ra and Ry treatment distributed along the positive direction. For the PC2 axis, the CK treatment still distributed along the negative direction. The Po distributed along the positive direction. The Ry scattered in both directions.
The f irst 12 carbon sources were analyzed based on the correlation coefficients of PC1 and PC2. The results showed that amino acids and carbohydrates were the main carbon sources for PC1 and PC2 (Table 3). Amino acids acted as the carbon source for 58.3% of the matter in PC1; carbohydrates were the carbon sources for 83.3% of the matter in PC2. Amino acids and carbohydrates were therefore determined as the main carbon source indicators of various cover crop straw returning treatments in winter.
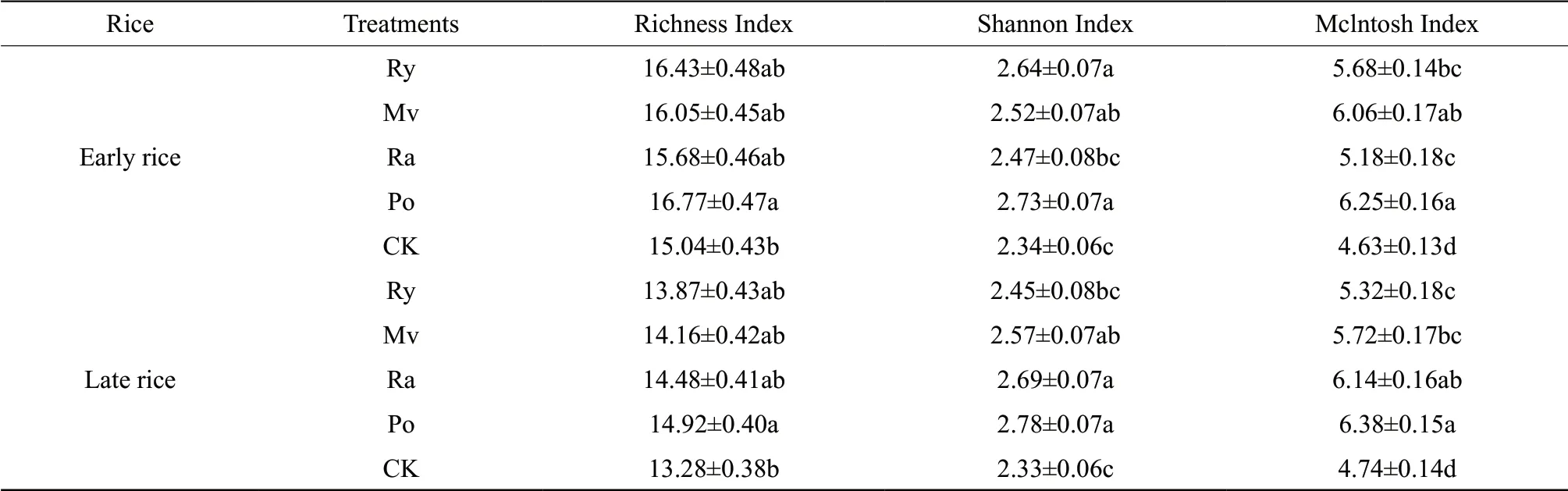
Table 2 Diversity Index of microf lora under different treatments in the rice maturity stage
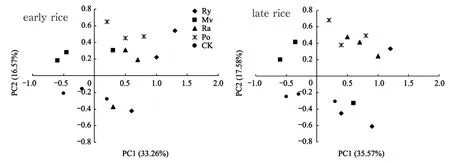
Fig. 2 Principal components of the microf lora’s metabolic diversity under different treatments in the rice maturity stage
The first 6 carbon sources were analyzed. Det- ailed carbon source codes are shown in Table 4. The results have proved that there is a strong correlation between the chemical properties of paddy soil and the utilization of carbon sources by rhizosphere microorganisms. As shown in Fig. 3, carbohydrates and carboxylic acids are mainly distributed on the right side of axis 1, whereas amino acids, esters, and some carbohydrates are on the left. A positive correlation can be observed between the right side of axis 1 (mainly carbohydrates and carboxylic acids) and soil pH value as well as the content of organic matter, total nitrogen and available nitrogen. The contents of available phosphorus and rapidly available potassium were the main environmental variables affecting amino acids and some carbohydrates.
4. Discussion
Previous research has proved that AWCD is an effective indicator of the carbon source utilization and metabolic activity of soil microorganisms[31]. ZHOU W X et al. found that straw returning could help improve the structure of the microbial communities as well as their metabolic capacity (characterized by AWCD) and functional diversity (Richness, Shannon and Mclntosh Index). In this paper, the straw returning of different winter cover crops had varied effects on the carbon source utilization of the microbial community in the rhizosphere soil. In comparison to CK, all treatments showed significant improvements in the AWCD, indicating that the straw returning of the exogenous winter cover crop was conducive to the carbon source utilization of the microorganisms in the rhizosphere soil. A possible reason is that the application of exogenous organisms (namely, the straw of winter cover crop) could provide suff icient carbon contents and nutrients for the soil microorganisms to enhance their microbial activity[11]. Among all the treatments, the returning of potato straw (Po) produced the highest AWCD, because the combination of potato straw (i.e. stems and leaves) with rice straw and proper fertilization could offer balanced nutrients (i.e. N, P, and K) and proper C/N, which could further facilitate the release of nutrients from the straw and accelerate microbial decomposition[32]. On the other hand, potato straw returning could change the dominant population of the microorganisms in the rhizosphere soil and increase their utilization rate of the tested carbon sources. Meanwhile, the amount of potato straw returned to the field, the contents of the straw (including rice straw), and the carbon contents returned to the field could also affect the carbon cycling and microbial activity in the soil[33]. In the maturity stage of early rice, the straw returning of ryegrass (Ry) and milk v etc h (Mv) also brought about increased AWCD, most probably because their straw was easy to decompose and release nutrients during the growth stage of early rice[23], effectively enhancing the utilization of the carbon source by the microorganisms. During the maturity stage of late rice, the straw returning of rape (Ra) also led to improved AWCD. A potential reason is that the rape straw was decomposing slowly in the growth stage of early rice and part of the straw didn’t decompose or release nutrients until the growth stage of late rice[22-23].
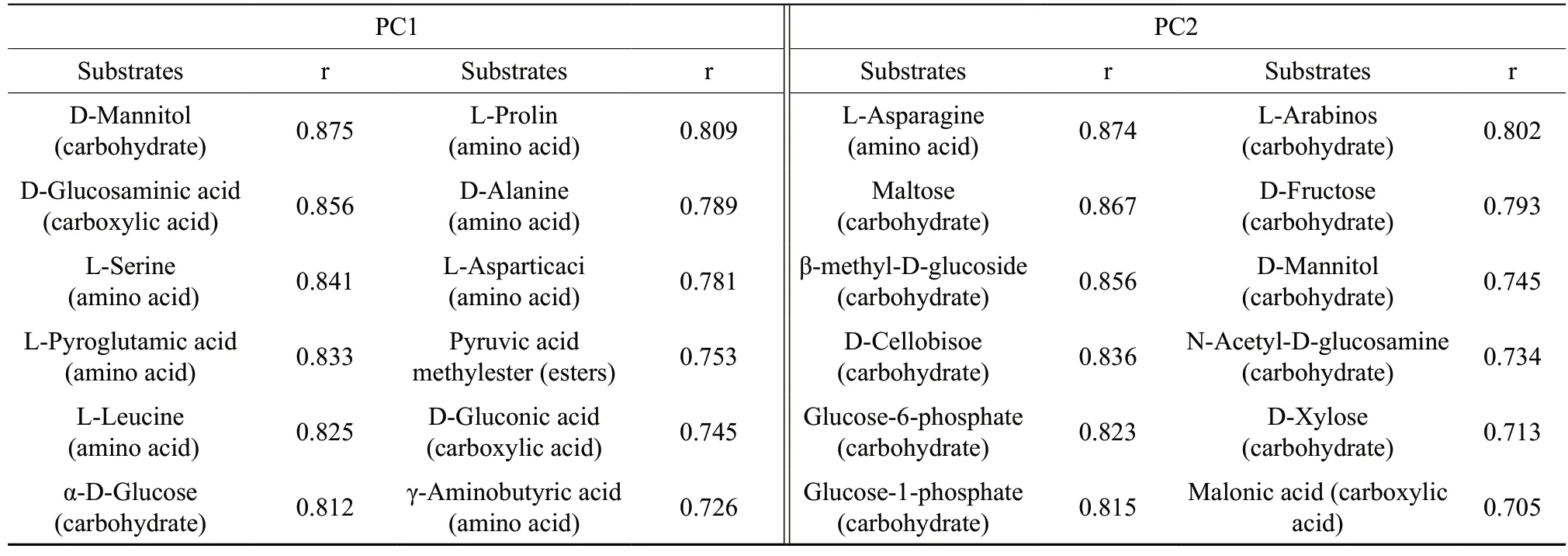
Table 3 Main substrates of high correlation with PC1 and PC2
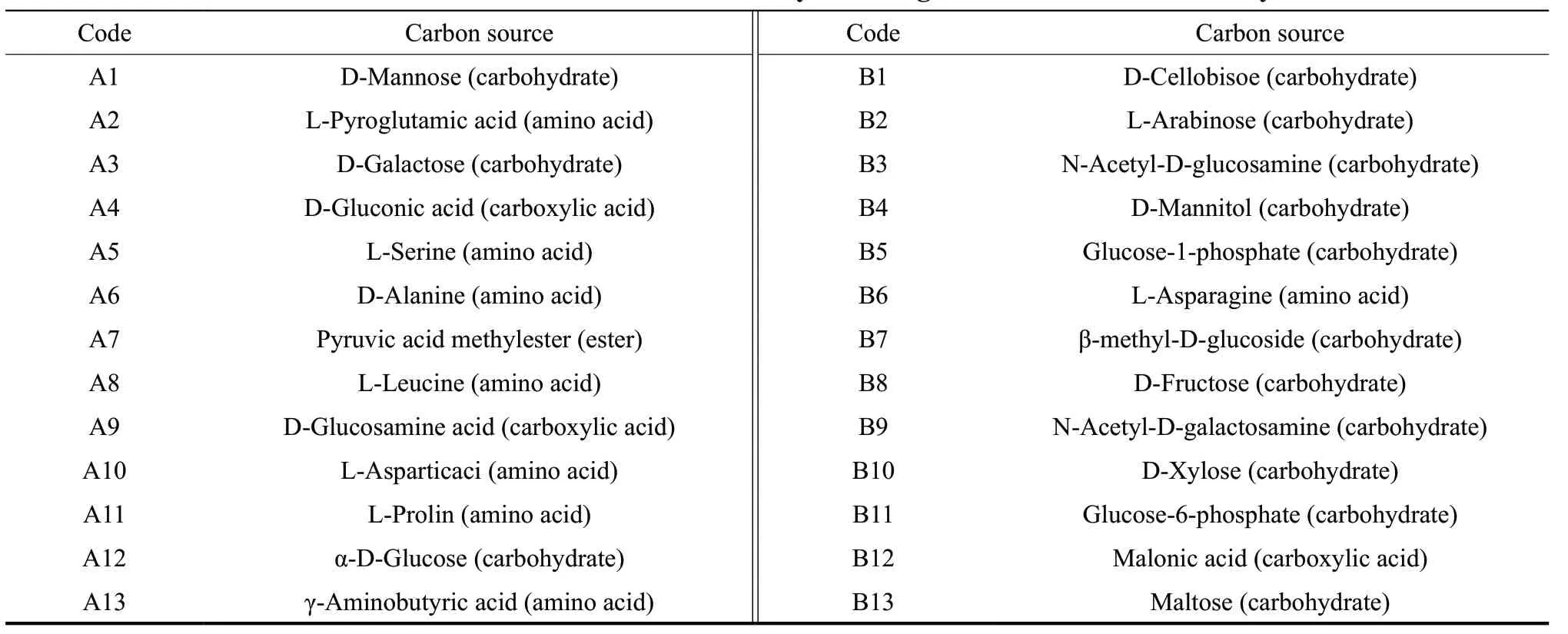
Table 4 Main carbon source codes utilized by microorganisms based on CCA analysis
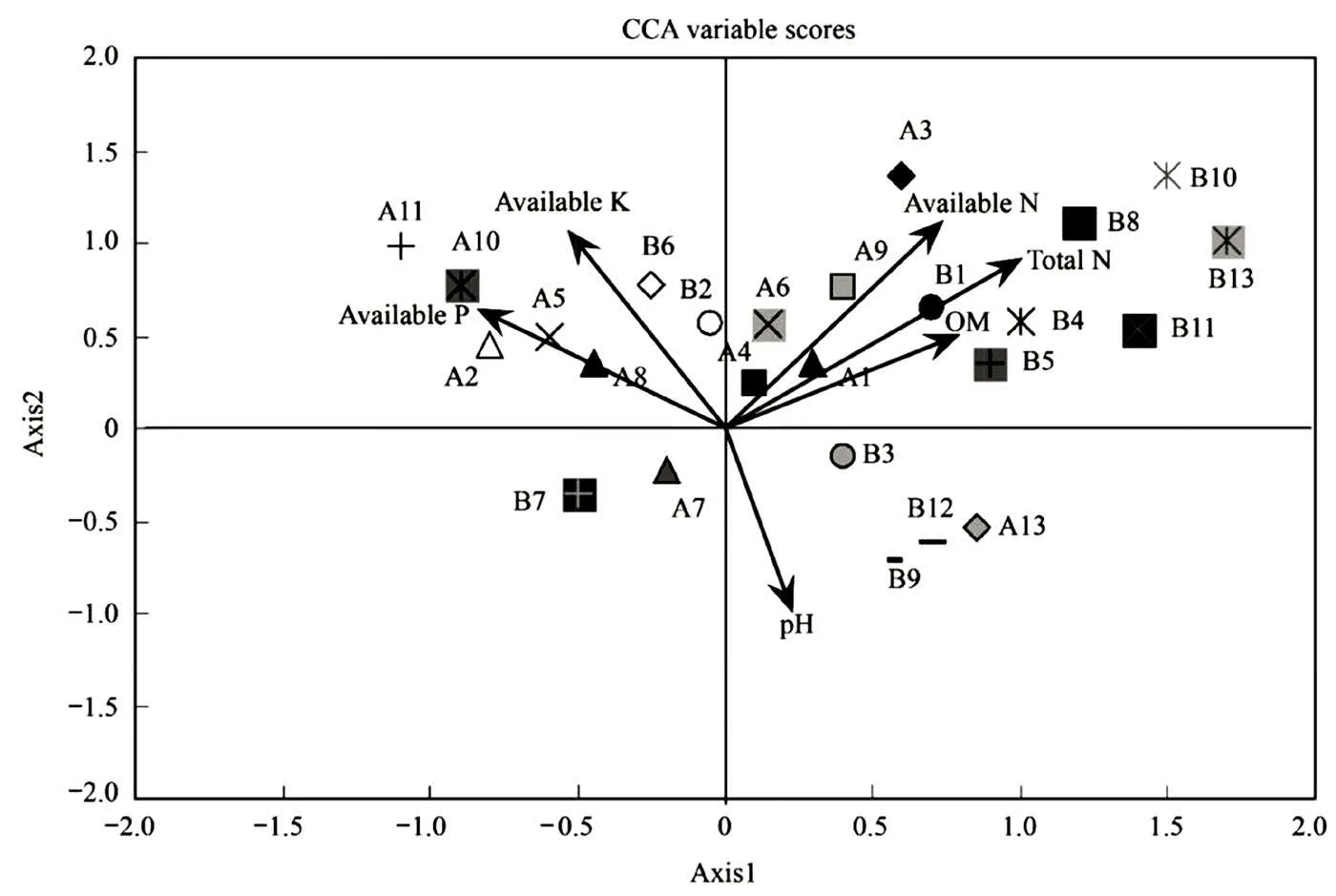
Fig. 3 Correlation between chemical properties of soil and carbon source utilization of microorganism
Previous research has demonstrated that the Richness, Shannon and Mclntosh Index are typical precursors to changes in the diversity of the microbial community in the soil[2]. In this research, the analytical results of these indexes indicated signif icant inf luences of different winter cover crops and straw returning on the diversity of the microf lora in rhizosphere soil. Differences were observed between the total number and the evenness of the microbial species under different treatments. The largest number of microbial species and the optimal evenness appeared in the Po treatment. This is because the combination of potato straw (i.e. stems and leaves) with rice straw and proper fertilization could offer balanced nutrients (i.e. N, P, and K) and proper C/N, further facilitating the release of nutrients and accelerating microbial decomposition. The supply of adequate energy and nutrients could stimulate the propagation[34]and metabolism of the microorganisms in the rhizosphere soil. Because of the abundant carbon content in the stems and leaves of potatoas well as in rice straw[33], this treatment could greatly enhance the carbon content in the soil and therefore produce higher carbon-related microbial activity. To conclude, Po is found being capable of improving the microbial activity of microorganisms in rhizosphere soil, strengthening carbon utilization (Fig. 1), and enhancing microbial diversity. Ry and Mv greatly increased the richness of the microbial species in the rhizosphere soil of early rice in contrast to CK, so did Ra and Mv for late rice. Ryegrass and milk vetch mainly decompose and release nutrients during the growth stage of early rice[35-36], while part of rape and milk vetch would not decompose until the growth stage of late rice[22-23]. This could be a potential reason for the difference in the results of the different treatments. The results of various indexes were ranked in different orders, which were closely related to the type of winter cover crop, the amount of straw returned to the paddy[22-23], the carbon content of the straw[19,33], the amount of carbon returned to the paddy[19-20,33], the decomposition velocity of the winter cover crop and rice straw in the soil[35-36], and the climate conditions in the growth stage of early rice and late rice[21]. According to the existing analysis of the principal components (PC), the carbon utilization of soil microorganisms showed a propensity to change with the type of treatment. The distribution of the samples along the PC axis is closely related to the carbon utilization of the microorganisms[12]. GARLAND et al.[2]found a correlation between microorganisms’ carbon utilization and the distribution of the samples on the PC axis. In this paper, only the first 2 principal components were analyzed. The results showed signif icant variations in the microorganisms’ carbon utilization under different treatments (Fig. 2). The carbon-related metabolic properties of the microorganisms in the rhizosphere soil are also subject to the content of the organic matter and the chemical properties of the soil. There are large quantities of carbohydrates and nutrient elements (i.e. N, P, and K) in winter cover crops, which could affect the content and structure of the organic matter, the content of some soil nutrients[20], the chemical properties of the soil, and the carbon utilization of the rhizosphere microorganisms (Fig. 3). Consequently, there would be changes in the microbial communities[37]. Furthermore, the soil aggregate provides an important microenvironment for the growth of the microorganisms. The number and quality of soil aggregates could affect the microorganisms in it. Soil aggregates are subject to the type of winter cover crops and straw returning[19]. Therefore, soil aggregates would also affect the properties of the microorganisms in the soil, causing changes in the carbon-based microbial metabolism. ZHOU W X et al.[14]found 9 carbon sources in their research on rice straw mulching, such as β-methyl-Dcarbohydrates, pyruvic acid methylester and D-Xylose. Amino acids and carbohydrates were the main carbon sources in this research (Table 3), probably because the application of exogenous organisms (namely, the straw of winter cover crops) gave rise to a higher content of amino acids and monosaccharides, providing suff icient carbon sources for the growth of the microorganisms[12]. There were large differences between the types of carbon sources under different treatments, indicating a signif icant inf luence of winter cover crop and straw returning on soil properties[20]. Meanwhile, correlations were observed between the type of treatment and the carbon utilization of the microorganisms in the soil (Fig. 3). The type of carbon-based sources utilized by the rhizosphere microorganisms under each treatment could be used to evaluate the inf luence of different winter cover crops on the microorganisms in rhizosphere soil[38-39].
5. Conclusion
In comparison to CK, all treatments showed signif icant improvements in AWCD, which means the straw returning of winter cover crops is conducive to maintaining the diversity of the microorganisms in rhizosphere soil. In particular, both the richness and evenness of the microbial species had a noticeable increase under the Po treatment (potato-doublecrop rice), quite a contrast to the decline of microbial evenness under CK. According to the analytical results, amino acids and carbohydrates were the main carbon sources utilized by the rhizosphere microorganisms, which also varied greatly in line with the type of winter cover crop. The treatment combining winter cover crop with straw returning could increase the diversity of the carbon utilization of the rhizosphere microorganisms in rice paddies. Canonical correspondence analysis (CCA) was applied to investigate the relations between the chemical properties of paddy soil and the carbon utilization of the rhizosphere microorganisms. These f indings have shown that most carbohydrates and carboxylic acids utilized by rhizosphere microorganisms are subject to soil pH value as well as the content of organic matter, total nitrogen and available nitrogen. The contents of available phosphorus and rapidly available potassium were proved to be the main environmental variables affecting amino acids and some carbohydrates.

