Regulatory role of calpain in neuronal death
Si-ying Cheng, Shu-chao Wang, Ming Lei Zhen Wang Kun Xiong
1 Xiangya Medical School, Central South University, Changsha, Hunan Province, China
2 Department of Anatomy and Neurobiology, School of Basic Medical Sciences, Central South University, Changsha, Hunan Province, China
Introduction
Three types of cell death have been identified (Green and Llambi, 2015; Chemaly et al., 2017). The first is apoptosis characterized by nuclear and cell shrinkage, chromatin condensation and fragmentation, and the formation of apoptotic bodies (Green and Llambi, 2015; Chemaly et al., 2017). Two apoptotic mechanisms have been reported: the intrinsic and extrinsic pathways (Chemaly et al., 2017). The extrinsic pathway is initiated by the binding of cell-surface death receptors to extracellular ligands, resulting in the formation of a death-inducing signaling complex. The intrinsic pathway is mediated by intracellular signals in the inner mitochondrial membrane (Chemaly et al., 2017). Following apoptotic signaling, many apoptotic proteins, such as caspases and B-cell lymphoma 2 (Bcl-2) family members, are activated or inhibited to participate in the regulation of apoptosis (Green and Llambi, 2015; Chemaly et al., 2017). The second type of cell death is autophagy, defined as the accumulation of two-membrane autophagic vacuoles in the cell plasma (Green and Llambi, 2015; Chemaly et al., 2017). The third type of cell death is necrosis, which features membrane rupture, release of cytoplasmic organelles, increased cytosolic calcium,and in flammation (Green and Llambi, 2015; Chemaly et al.,2017). In recent years, increasing evidence has indicated that necrosis can be molecularly controlled, and therefore it has been rede fined as “regulated necrosis” (Galluzzi et al., 2014;Pasparakis and Vandenabeele, 2015). Regulated necrosis can be divided into cell-death modalities, such as receptor interacting protein (RIP)-dependent necroptosis, mitochondrial permeability transition (MPT)-dependent necrosis, and pyroptosis and poly (ADP-ribose) polymerase 1 (PARP1)-mediated parthanatos, which function in the pathogeneses of many nervous system diseases and during acute cellular damage(Pasparakis and Vandenabeele, 2015; Xiong et al., 2016; Chemaly et al., 2017; Shang et al., 2017). Apoptosis, autophagy and regulated necrosis are de fined as programmed cell death by many researchers (Pasparakis and Vandenabeele, 2015;Prasad and Kaestner, 2017; Thornton et al., 2017).
Numerous previous studies reported that calpain plays an important role in programmed cell death in nervous system diseases, such as stroke, Alzheimer’s disease and Huntington’s disease (Saatman et al., 1996b; Bartus, 1997; Bartus et al., 1998; James et al., 1998; Marklund et al., 2006; Pandey et al., 2016). Calpains are a group of calcium-dependent neutral proteases, which are ubiquitously expressed in different tissue types and organisms (Saatman et al., 1996a; Suzuki et al., 2014; M?rtensson et al., 2017). Fifteen members of the calpain family have been identified to date (Curcio et al., 2016). Calpain family members can be classified into classical and non-classical types based on their domain IV structure (Singh et al., 2014; Curcio et al., 2016). Classical calpains (1, 2, 3, 8, 9, 11, 12, and 14) contain a penta-EF hand in domain IV that binds to calcium. Three of the eight classical calpains (calpain-1, 2, and 9) dimerize with the calpain small subunit in mammals (Singh et al., 2014;Curcio et al., 2016). Non-classical calpains (5, 6, 7, 10, 13,and 15) lack the penta-EF hand in domain IV and cannot bind to the calpain small subunit (Singh et al., 2014; Curcio et al., 2016). The best characterized calpains in the central nervous system are two distinct, heterodimeric subtypes:μ-calpain and m-calpain, also known as calpain-1 and calpain-2, although many other calpains (calpain-3, calpain-5 and calpain-10) are also expressed in the nervous system(Singh et al., 2014). The activation of calpain-1 requires 3—50 μM calcium and the activation of calpain-2 requires 0.4—0.8 mM calcium (Curcio et al., 2016). Inactive calpains exist in the cytoplasm and translocate to the membrane when exposed to increased cellular calcium levels. Then, calpain,combined with calcium, is activated in the presence of phospholipids. Finally, the activated calpain degrades substrate proteins, such as spectrin, calcium-dependent transcription factor, caspase family members, Bcl-2 family members, RIP,and AIF, at membranes or in the cytosol after release from the membranes (Singh et al., 2014; Suzuki et al., 2014). Although calpain cleaves preferred sequences in association with preferred tertiary structures of substrates, the substrate speci ficity de fies complete classi fication (Lynch and Gleichman, 2007). The complex involvement of calpains in vital cell functions suggests that the dysfunction of calpain may cause the excessive degradation or accumulation of cellular proteins, leading to various cellular damage and pathological conditions (Bartus, 1997, Suzuki et al., 2004). In this review,we summarize the current knowledge of the two main calpain isoforms, calpain-1 and calpain-2, in relation to different models of cell death and the roles of calpains with their speci fic substrates, which are important for the induction or repression of different models of cell death, such as apoptosis, autophagy and regulated necrosis.
Calpain and Apoptosis
Calpain and caspase family members
Caspase-dependent apoptosis is one of the main causes of neuronal death in neurodegeneration (Wang et al., 2016c;Ge et al., 2017). Calpains affect a number of proteins in the caspase family (Bakshi et al., 2005). For example, it has been widely veri fied that the activation of caspase-12 is mediated by calpains in the nervous system (Martinez et al., 2010; Imai et al., 2014). During neuronal death induced by salinomycin, activated calpain-1 and calpain-2 cleave and activate caspase-12,then activate caspase-9 and its effector protein caspase-3(Gorman et al., 2000; Boehmerle and Endres, 2011). Calpains also mediate the activity of caspase-3 through other pathways.Yamada et al. (2012) reported that calpain-1 knockout neurons had less caspase-3 and apoptosis activity than heterozygous neurons, possibly because calpain-1 knockout increased the activity of X-linked inhibitor of apoptosis protein (XIAP),a physiological inhibitor of caspase-3. XIAP is degraded by calpain-1, and calpain-1 deficiency enhanced the inhibitory effect of XIAP on caspase-3 (Yamada et al., 2012). Although many researchers agree that calpains enhance caspase activity, other studies claim that calpain-2 can also cleave and block the activation of caspases in different cell types (such as MCF-7 cells and SH-SY5Y cells); furthermore, various effects have been reported in the same cell types under different apoptotic stimulations (such as staurosporine, hydrogen peroxide and serum starvation) (Chua et al., 2000; Tan et al.,2006). Therefore, calpain-2 may act as a negative regulator of caspase processing and apoptosis (Chua et al., 2000). Chua et al. (2000) reported that activated calpain-2 cleaved caspase-9,which is incapable of activating caspase-3, and prevented subsequent cytochrome c release in cells. In that study, the short pro-domain effectors, caspase-7/8/9, were calpain-specific cleavage sites (Chua et al., 2000). In summary, it is possible that calpains have opposite roles in apoptosis and their effects on caspases may be different (Wang et al., 2012).
Effect of calpain on Bcl-2 family members
Bcl-2 protein was first discovered by the analysis of chromosomal translocation in a B cell follicular lymphoma (Youle and Strasser, 2008; Kvansakul and Hinds, 2015; Wu et al.,2016). Subsequently, other Bcl-2 family proteins, such as Bax,Bak, Bid, and Bcl-xL, were identi fied (Kvansakul and Hinds,2015). The Bcl-2 family proteins are essential in the mitochondrial apoptotic pathway, because they directly regulate the permeability of the outer mitochondrial membrane. A channel is formed on the outer mitochondrial membrane by Bax and Bad, through which cytochrome c, apoptosis protease activating factor 1, and the deoxyribonucleotide triphosphate complex, enter the cytoplasm, which activates caspase-9 and caspase-3 and induces apoptosis (Bleicken et al., 2013). Activated calpains cleave the N-terminal of Bax into a pro-apoptotic 18-kDa fragment, stimulating the release of cytochrome c and apoptosis (Gao and Dou, 2000). Similarly, under the stimulus of apoptotic signals such as Shigella infection, ischemia/reperfusion injury, and DNA-damaging agents, calpain-1 splices Bid into t-Bid, which has a better binding affinity for mitochondrial membranes, increases membrane permeability and produces oligomers that regulate apoptosis (Chen et al.,2001; Mandic et al., 2002; Andree et al., 2014).
Effect of calpain on AIF
AIF translocates from the mitochondria to the cytoplasm and into the nucleus when exposed to apoptotic signaling (Sevrioukova, 2011). In the inner membrane of mitochondria, AIF is truncated by calpains and this truncated AIF (tAIF) enters the cytoplasm through a permeability transition pore. It then activates caspase-9 and induces the endogenous apoptotic pathway by initiating chromatin condensation and DNA fragmentation (Ghavami et al., 2014). Yamada et al. (2012)reported that during neuronal apoptosis induced by ischemia,the translocation of AIF from the mitochondria to the cytosol was decreased in calpain-1 knockout neurons. As a result,apoptosis was inhibited. Similarly, in retinitis pigmentosa, the inhibition of calpain-1 inhibited AIF activation and decreased retinal degeneration and photoreceptor apoptosis (Ozaki et al., 2013). Heat shock protein 70 is associated with AIF and sustains the stability of AIF to prevent apoptosis; however,calpain-1 degrades heat shock protein 70 to maintain the transport of AIF from the mitochondria to the cytoplasm or nucleus (Matsumori et al., 2005).
Effect of calpain on cyclin-dependent kinase 5 (CDK5)
Cyclin-dependent kinases (CDKs) are a family of protein kinases first discovered for their roles in regulating the cell cycle (Bramanti et al., 2015). They are also involved in regulating transcription, apoptosis, and the differentiation of nerve cells (Arisan et al., 2014; Bramanti et al., 2015). They are present in all known eukaryotes, and their regulatory function in the cell cycle has been evolutionarily conserved(Bramanti et al., 2015). A CDK binds to cyclin, a regulatory protein. Without cyclin, CDK has little kinase activity; only the cyclin-CDK complex is an active kinase. Therefore, the activity of CDKs is regulated by phosphorylation and by binding inhibitory proteins termed cyclin-dependent kinase inhibitors (Bramanti et al., 2015). Inactive CDK5 monomer is only functional when attached to regulatory subunit P35 or P25. P35 is hydrolyzed by calpain into P25 and P10. P25 activates CDK5, forming a CDK5-P25 complex, which in-activates myocyte enhancer factor, an important survival factor for dopamine neurons (Mount et al., 2013; Zhang et al., 2016). The CDK5-P25 complex upregulates P53 expression, which activates caspase-3 and induces apoptosis (Alvira et al., 2008). Furthermore, the phosphorylation of NR2A, a subunit of N-methyl-D-aspartate receptors (NMDAR), is increased by the calpain-P35/P25-CDK5 pathway, which leads to the increased expression of functional NMRAR and calcium overload, resulting in glutamate-induced retinal neuronal apoptosis (Miao et al., 2012).
Calpain and Autophagy
Autophagy is a physiological process that digests extra substances in the cytoplasm by the autophagosome lysosomal pathway (Yang and Klionsky, 2010; Ohsumi, 2014). The critical role of autophagy is removing damaged intracellular organelles/misfolded proteins in neurons (Yang and Klionsky,2010; Ohsumi, 2014). A number of studies have indicated that calpains interfere with autophagic pathways in the nervous system (Williams et al., 2008; Zhang et al., 2009; Menzies et al., 2015). Ataxin-3, the disease protein in Machado-Joseph disease, was predominately hydrolyzed by calpain-1/2 (Weber et al., 2017). The disturbance of calpain-1/2 inhibition might promote the formation of ataxin-3 (Hubener et al.,2013), ultimately leading to the inhibition of autophagy (Watchon et al., 2017). In recent years, more than 36 subtypes of autophagy-related genes (Atg) involved in autophagy have been identified (Ohsumi, 2014). The early formation of autophagosomes requires multiple Atg complexes and Beclin-1(Russo et al., 2011; Chinskey et al., 2014). Based on a study by Chinskey et al. (2014), after retinal injury, Atg-5 is inactivated by calpain-1, which attenuates autophagic activity in photoreceptor neurons (Chinskey et al., 2014). Furthermore,calpains degrade beclin-1 and inhibit autophagy (Russo et al.,2011). Further studies indicated that under some autophagic conditions, reduced intracellular calcium levels might serve as a signal to inhibit calpain-1 activity, which in turn might activate autophagic activity by enhancing the levels of autophagic signaling molecules such as Atg-5, beclin-1 and the Atg-12-Atg-5 complex (Cecconi and Levine, 2008). Lysosome rupture is an essential element in cell death (Rodriguez-Muela et al.,2015). A previous study reported that permeability of the lysosome membrane is regulated by calpain by many mechanisms(Geronimo-Olvera et al., 2017). Calpain cleaves lysosome associated membrane permeabilization 2 at the lysosome membrane and mediates lysosomal membrane permeabilization, which may lead to lysosomal dysfunction and decreased autophagy (Villalpando Rodriguez and Torriglia, 2013; Rodriguez-Muela et al., 2015; Geronimo-Olvera et al., 2017).
Studies have also suggested that calpains are responsible for the conversion of the autophagic pathway to the apoptotic pathway (Youse fiet al., 2006; Chung et al., 2015; Song et al., 2017). During cell death in hippocampal neural stem cells induced by insulin withdrawal, the inhibition of calpain-2 leads to a preference for autophagic cell death, and an increase of calpain-2 expression converts the autophagic pathway to the apoptotic pathway (Chung et al., 2015). This finding indicates that autophagy might have a close connection with apoptosis, as autophagy influences apoptosis through the degradation of caspase-8 or -9. Furthermore,apoptosis affects the autophagic flux by cleaving autophagy molecules, such as Beclin-1 or Atg-5 (Chung et al., 2015).Other studies reported that when Atg-5 is cleaved by calpain its autophagy activity is inhibited (Youse fiet al., 2006;Del Bello et al., 2013). In cells exposed to apoptotic stimuli,cleaved Atg5 translocates into the mitochondria and combines with Bcl-xL, thereby inducing apoptosis (Zhou et al.,2011; Del Bello et al., 2013).
Calpain and Regulated Necrosis
Calpain and parthanatos
A series of genotoxic stresses, such as alkylating agents and N-methyl-N-nitro-N-nitrosoguanidine, have been veri fied to result in cell necrosis associated with the activation of PARP-1, termed parthanatos (Wang et al., 2009; Harbison et al.,2011; Muller et al., 2014). Parthanatos characterized by overactive PARP-1 is caused by metabolic disturbance, such as the excessive consumption of nicotinamide adenine dinucleotide and adenosine triphosphate (van Wijk and Hageman,2005). Baritaud et al. (2010) further uncovered the specific molecular mechanism by which calpains regulate necrosis.Acute DNA damage activates PARP-1 in the nucleus. When activated, PARP-1 is transferred to the mitochondrial membrane to activate calpain-1, which is truncated and activates AIF. In the nucleus, tAIF, Histone H2AX and cyclophilin break down the DNA into large fragments (Baritaud et al.,2010). Calpain-1 mediates AIF release from mitochondria and necrosis through a mechanism that is distinct from apoptosis but which is caspase-independent. The reason might be explained, because apoptosis is abrogated by the cleavage of apoptotic effectors, such as caspases, by calpain (Moubarak et al., 2007). Furthermore, because calpain-1 directly cleaves AIF to tAIF, calpains might also cleave BID to t-BID, which facilitates BAX activation and subsequent AIF mediated parthanatos (Cabon et al., 2012). In addition, PARP-1 is the substrate of calpains and the activation of PARP-1 requires the activation of calpains (Sacca et al., 2016). Therefore, PARP-1 and calpain-1 might act in concert following injury to induce AIF-mediated necrosis (Chiu et al., 2012).
Calpain and necroptosis
Necroptosis, a RIP-mediated programmed form of necrosis induced by tumor necrosis factor α, is regulated by calpain(Bollino et al., 2015; Pasparakis and Vandenabeele, 2015)(Figure 1). Calpains degrade c-Jun N-terminal kinase (JNK)inhibitor JNK-interacting protein-1, and then activate JNK-1, which increases the expression of RIP-1. RIP-1 then binds to RIP-3 and initiates necroptosis mechanisms, including second mitochondria-derived activator of caspase/direct IAP-binding protein with low PI and AIF release from mitochondria (Bollino et al., 2015). Our previous studies reported that in vitro elevated hydrostatic pressure or oxygen-glucose deprivation induced the necroptosis of retinal ganglion cells(RGC-5 cell line); thus, calpains play an important role in mediating necroptosis via tAIF (Shang et al., 2014; Chen et al., 2016). Our recent research also found that Pin1 interacts with calpastatin, an endogenous calpain inhibitor, to modulate the activity of calpain 2 in the presence of excessive glutamate, thereby causing tAIF mediated necroptosis in primary rat retinal neurons, the RGC-5 cell line, and the ganglion cell layer and inner nuclear layer of the rat retina (our unpublished data). Although necroptosis and parthanatos share a common necrotic effector, AIF, they represent two independent and distinct pathways that regulate necrosis (Sosna et al., 2014). Necroptosis and parthanatos are induced by tumor necrosis factor α and N-methyl-N-nitro-N-nitrosoguanidine,respectively. In contrast to parthanatos that does not depend on caspase activity, the regulation of necroptosis depends on caspase-8 activity (Sosna et al., 2014).
Calpain and other regulated necroses, including pyroptosis and MPT mediated regulated necrosis
Pyroptosis is a form of inflammatory cell necrosis that requires the activation of caspase-1 (Fink and Cookson, 2005;He et al., 2015). During pyroptosis, caspase-1 is activated by the pyroptosome, which is composed of dimers of the adaptor protein apoptosis-associated speck protein containing a CARD or caspase activation and recruitment domain (Soong et al., 2012; He et al., 2015). Although there are numerous caspase-1 activation pathways, the downstream pathway results in pyroptosis; the apoptosis pathway is associated with caspases-3 and-7, but not caspase-1 (Soong et al., 2012; Sun et al., 2016). There are two types of sensory receptors involved in pyroptosis, Toll-like receptors and Nod-like receptors, which sense danger signals (Soong et al., 2012). Chun et al. (2009)reported that Toll-like receptor 2 stimulation results in increased calcium flux. Subsequently, the activation of calcium dependent calpains is targeted by caspase-1, which cleaves the transmembrane proteins occludin and E-cadherin (Chun and Prince, 2009; Soong et al., 2012), resulting in pyroptosis.
The MPT pore is an inducible inner mitochondrial membrane pore involved in apoptotic and necrotic death(Douglas and Baines, 2014; Lu et al., 2014). The formation of MPT is regulated by calpain-mediated proteolytic events(Arrington et al., 2006). Under MPT conditions, osmosis forces a large volume of water into the mitochondrial matrix, resulting in the release of various apoptotic activators such as Bcl-2 family members, into the cytoplasm (Oh and Lim, 2006). MPT also triggers a pathway that regulates necrosis and which is regulated by a key regulatory molecule,cyclophilin D (Lu et al., 2014). Subsequent studies found that MPT formation is an important upstream mediator of integrin αIIbβ3inactivation and that calpain activation might activate integrin through talin cleavage (Liu et al., 2013).
However, although pyroptosis and MPT regulated necrosis were con firmed in neurons, correlations between calpain and the two types of necrosis are lacking. Therefore, studies are needed to determine whether calpain has a relationship with cell necrosis in nervous system diseases.
Perspective
In addition to the above mentioned substrates, other studies reported that the direct targets of calpains include most major glutamate receptors, such as α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptors, NMDA receptors,and metabotropic glutamate receptors (Dong et al., 2004;Wu et al., 2007; Curcio et al., 2016; Wang et al., 2018). By the proteolysis of these receptors and associated proteins, calpains may modulate the activity of glutamate synapses (Curcio et al., 2016). As a result, calpain proteolysis in neurons might result in pathological events such as excitotoxicity,but also neuroprotective roles in cell survival and synaptic transmission (Wu et al., 2007; Doshi and Lynch, 2009).Calpain-1 and calpain-2 play opposite roles in cell survival and death (Wang et al., 2016b). Activation of synaptic NMDAR-coupled calpain-1 is neuroprotective, while activation of extrasynaptic NMDAR-coupled calpain-2 is neurodegenerative (Wang et al., 2016b). Calpain-1 is involved in Akt and extracellular signal-regulated kinase mediated cell survival. Activated calpain-2 cleaves and inactivates STEP,resulting in p38-induced cell death (Wang et al., 2016b).This provides new insights into the mechanism of calpain mediated cell death. Based on these previous findings, further studies are needed to explore the detailed mechanisms of calpain mediated cell survival or death.
For most calpain mediated disorders, inhibitors are the first and logical therapeutic choice (Ono et al., 2016). To achieve a potential therapy, it is critical to produce speci fic molecules that have the correct physical chemistry to function as drugs for the treatment of neural diseases (Bartus et al., 1999; Laurer and McIntosh, 2001; Wang et al., 2016a). Many previous studies have suggested that calpain inhibitors are useful for treating brain injuries by preventing neuronal loss and improving behavior (Saatman et al., 1996b; Bartus, 1997; James et al., 1998; Marklund et al., 2006; Pandey et al., 2016). Furthermore, some calpain inhibitors are being tested in clinical trials; AbbVie has initiated a Phase I clinical trial with an orally active non-selective calpain inhibitor for the treatment of Alzheimer’s disease (Wang et al., 2016b). However, to our knowledge, many of the calpain inhibitors are nonspeci fic and target other proteases. Therefore, understanding the calpain substrates and the speci fic pathways of calpain mediated cell death are key concerns, especially when considering potential off-target effects (Ono et al., 2016). There is also a need to develop speci fic and bene ficial therapeutic calpain inhibitors.
Conclusions
A preliminary understanding of the regulatory role of calpains in programmed neuronal death has been reached. Calpains play an important role in apoptosis, autophagy, and regulate necrosis (Table 1). Therefore, calpain is a promising therapeutic target for neurological diseases. Speci fic inhibitors of calpains may bring new insights for the treatment of related diseases. However, because of potential species-speci fic differences, there have been few investigations regarding the mechanisms involved in human nervous system diseases, which are different from cells or animal models of disease. Therefore, different potential risks, including calpain dysfunction, might lead to the progression of one type of nervous disease. Thus, future pathogenic mechanisms,not only the deregulated calpain mediated central nervous system dysfunction, should be investigated further to understand the progression of nervous diseases.
Author contributions:SYC, SCW and ML wrote the first draft of the paper.KX and ZW coordinated the work and revised the paper. All authors approved the final version of the paper.
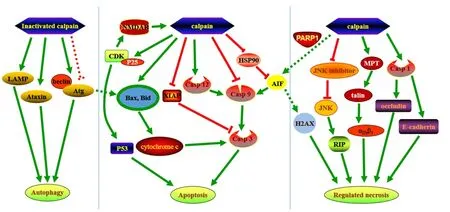
Figure 1 Hypothetical mechanisms of calpain in neuronal death.
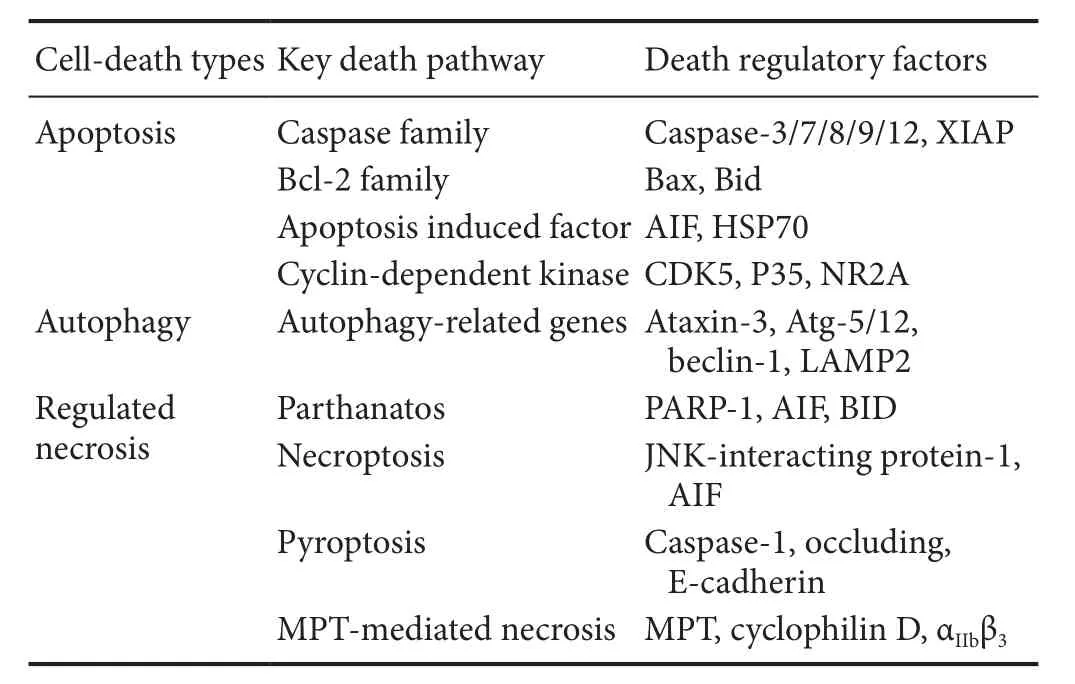
Table 1 Specific substrates of calpains that induce or repress cell death
Con flicts of interest:None declared.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81571939 & 81772134; the Wu Jie-Ping Medical Foundation of the Minister of Health of China, No. 320.6750.14118; the Natural Science Foundation of Hunan Province of China, No. 2015JJ2187;the Teacher Research Foundation of Central South University of China, No.2014JSJJ026. None of the funding bodies played any role in the study other than to provide funding.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Alvira D, Ferrer I, Gutierrez-Cuesta J, Garcia-Castro B, Pallas M, Camins A(2008) Activation of the calpain/cdk5/p25 pathway in the girus cinguli in Parkinson’s disease. Parkinsonism Relat D 14:309-313.
Andree M. (2014) BID-dependent release of mitochondrial SMAC dampens XIAP-mediated immunity against Shigella. EMBO J 33:2171-2187.
Arisan ED, Obakan P, Coker-Gurkan A, Calcabrini A, Agostinelli E, Unsal NP(2014) CDK inhibitors induce mitochondria-mediated apoptosis through the activation of polyamine catabolic pathway in LNCaP, DU145 and PC3 prostate cancer cells. Curr Pharm Design 20:180-188.
Arrington DD, Van Vleet TR, Schnellmann RG (2006) Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol 291:C1159-1171.
Bakshi A, Keck CA, Koshkin VS, LeBold DG, Siman R, Snyder EY, McIntosh TK (2005) Caspase-mediated cell death predominates following engraft-ment of neural progenitor cells into traumatically injured rat brain. Brain Res 1065:8-19.
Baritaud M, Boujrad H, Lorenzo HK, Krantic S, Susin SA (2010) Histone H2AX: the missing link in AIF-mediated caspase-independent programmed necrosis. Cell Cycle 9:3166-3173.
Bartus RT (1997) The calpain hypothesis of neurodegeneration: evidence for a common cytotoxic pathway. Neuroscientist 3:314-327.
Bartus RT, Chen EY, Lynch G, Kordower JH (1999) Cortical ablation induces a spreading calcium-dependent, secondary pathogenesis which can be reduced by inhibiting calpain. Exp Neurol 155:315-326.
Bartus RT, Dean RL, Mennerick S, Eveleth D, Lynch G (1998) Temporal ordering of pathogenic events following transient global ischemia. Brain Res 790:1-13.
Bleicken S, Landeta O, Landajuela A, Basanez G, Garcia-Saez AJ (2013)Proapoptotic Bax and Bak proteins form stable protein-permeable pores of tunable size. J Biol Chem 288:33241-33252.
Boehmerle W, Endres M (2011) Salinomycin induces calpain and cytochrome c-mediated neuronal cell death. Cell Death Dis 2:e168.
Bollino D, Balan I, Aurelian L (2015) Valproic acid induces neuronal cell death through a novel calpain-dependent necroptosis pathway. J Neurochem 133:174-186.
Bramanti V, Grasso S, Tomassoni D, Traini E, Raciti G, Viola M, Li Volti G,Campisi A, Amenta F, Avola R (2015) Effect of growth factors and steroid hormones on heme oxygenase and cyclin D1 expression in primary astroglial cell cultures. J Neurosci Res 93:521-529.
Cabon L, Galan-Malo P, Bouharrour A, Delavallee L, Brunelle-Navas MN,Lorenzo HK, Gross A, Susin SA (2012) BID regulates AIF-mediated caspase-independent necroptosis by promoting BAX activation. Cell Death Differ 19:245-256.
Cecconi F, Levine B (2008) The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell 15:344-357.
Chemaly ER, Troncone L, Lebeche D (2017) SERCA control of cell death and survival. Cell Calcium doi: 10.1016/j.ceca.2017.07.001.
Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA (2001) Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem 276:30724-30728.
Chen S, Yan J, Deng HX, Long LL, Hu YJ, Wang M, Shang L, Chen D, Huang JF, Xiong K (2016) Inhibition of calpain on oxygen glucose deprivation-induced RGC-5 necroptosis. J Huazhong Univ Sci Technolog Med Sci 36:639-645.
Chinskey ND, Zheng QD, Zacks DN (2014) Control of photoreceptor autophagy after retinal detachment: the switch from survival to death. Invest Ophth Vis Sci 55:688-695.
Chiu SC, Huang SY, Tsai YC, Chen SP, Pang CY, Lien CF, Lin YJ, Yang KT(2012) Poly (ADP-ribose) polymerase plays an important role in intermittent hypoxia-induced cell death in rat cerebellar granule cells. J Biomed Sci 19:29.
Chua BT, Guo K, Li P (2000) Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J Biol Chem 275:5131-5135.
Chun J, Prince A (2009) TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe 5:47-58.
Chung KM, Park H, Jung S, Ha S, Yoo SJ, Woo H, Lee HJ, Kim SW, Kim EK, Moon C, Yu SW (2015) Calpain determines the propensity of adult hippocampal neural stem cells to autophagic cell death following insulin withdrawal. Stem Cells 33:3052-3064.
Curcio M, Salazar IL, Mele M, Canzoniero LM, Duarte CB (2016) Calpains and neuronal damage in the ischemic brain: the swiss knife in synaptic injury. Prog Neurobiol 143:1-35.
Del Bello B, Toscano M, Moretti D, Maellaro E (2013) Cisplatin-induced apoptosis inhibits autophagy, which acts as a pro-survival mechanism in human melanoma cells. PLoS One 8:e57236.
Dong YN, Waxman EA, Lynch DR (2004) Interactions of postsynaptic density-95 and the NMDA receptor 2 subunit control calpain-mediated cleavage of the NMDA receptor. J Neurosci 24:11035-11045.
Doshi S, Lynch DR (2009) Calpain and the glutamatergic synapse. Front Biosci (Schol Ed) 1:466-476.
Douglas DL, Baines CP (2014) PARP1-mediated necrosis is dependent on parallel JNK and Ca(2)(+)/calpain pathways. J Cell Sci 127:4134-4145.
Fink SL, Cookson BT (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73:1907-1916.
Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A (2014) Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol 35:24-32.
Gao G, Dou QP (2000) N-terminal cleavage of bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death. J Biol Chem 80:53-72.
Ge X, Hu C, Guo Q, Li W, Zhao Y, Yang W, Wang Y, Li P, Gao Y, Huang Q(2017) Investigation of the expression of apoptosis-inducing factor-mediated apoptosis in Hirschsprung’s disease. Neuroreport 28:571-578.
Geronimo-Olvera C, Montiel T, Rincon-Heredia R, Castro-Obregon S,Massieu L (2017) Autophagy fails to prevent glucose deprivation/glucose reintroduction-induced neuronal death due to calpain-mediated lysosomal dysfunction in cortical neurons. Cell Death Dis 8:e2911.
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M,Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M,Owji AA, Los MJ (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24-49.
Gorman AM, Ceccatelli S, Orrenius S (2000) Role of mitochondria in neuronal apoptosis. Dev Neurosci-Basel 22:348-358.
Green DR, Llambi F (2015) Cell Death Signaling. Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a006080.
Harbison RA, Ryan KR, Wilkins HM, Schroeder EK, Loucks FA, Bouchard RJ, Linseman DA (2011) Calpain plays a central role in 1-methyl-4-phenylpyridinium (MPP+)-induced neurotoxicity in cerebellar granule neurons. Neurotox Res 19:374-388.
He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ,Han J (2015) Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res 25:1285-1298.
Hubener J, Weber JJ, Richter C, Honold L, Weiss A, Murad F, Breuer P,Wullner U, Bellstedt P, Paquet-Durand F, Takano J, Saido TC, Riess O,Nguyen HP (2013) Calpain-mediated ataxin-3 cleavage in the molecular pathogenesis of spinocerebellar ataxia type 3 (SCA3). Hum Mol Genet 22:508-518.
Imai T, Kosuge Y, Endo-Umeda K, Miyagishi H, Ishige K, Makishima M,Ito Y (2014) Protective effect of S-allyl-L-cysteine against endoplasmic reticulum stress-induced neuronal death is mediated by inhibition of calpain. Amino Acids 46:385-393.
James T, Matzelle D, Bartus R, Hogan EL, Banik NL (1998) New inhibitors of calpain prevent degradation of cytoskeletal and myelin proteins in spinal cord in vitro. J Neurosci Res 51:218-222.
Kvansakul M, Hinds MG (2015) The Bcl-2 family: structures, interactions and targets for drug discovery. Apoptosis 20:136-150.
Laurer HL, McIntosh TK (2001) Pharmacologic therapy in traumatic brain injury: update on experimental treatment strategies. Curr Pharm Design 7:1505-1516.
Liu F, Gamez G, Myers DR, Clemmons W, Lam WA, Jobe SM (2013) Mitochondrially mediated integrin alphaIIbbeta3 protein inactivation limits thrombus growth. J Biol Chem 288:30672-30681.
Lopatniuk P, Witkowski JM (2011) Conventional calpains and programmed cell death. Acta Biochim Pol 58:287-296.
Lu CC, Huang BR, Liao PJ, Yen GC (2014) Ursolic acid triggers nonprogrammed death (necrosis) in human glioblastoma multiforme DBTRG-05MG cells through MPT pore opening and ATP decline. Mol Nutr Food Res 58:2146-2156.
Lynch DR, Gleichman AJ (2007) Picking up the pieces: the roles of functional remnants of calpain-mediated proteolysis. Neuron. 53: 317-319.
Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S,Shoshan MC (2002) Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol 22:3003-3013.
Marklund N, Bakshi A, Castelbuono DJ, Conte V, McIntosh TK (2006)Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr Pharm Design 12:1645-1680.
M?rtensson LB, Blom CL, Dahlin LB (2017) Ca2+involvement in activation of extracellular- signal-regulated-kinase 1/2 and m-calpain after axotomy of the sciatic nerve. Neural Regen Res 12:623-628.
Martinez JA, Zhang Z, Svetlov SI, Hayes RL, Wang KK, Larner SF (2010)Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis 15:1480-1493.
Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J (2005) Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab 25:899-910.
Menzies FM, Garcia-Arencibia M, Imarisio S, O’Sullivan NC, Ricketts T,Kent BA, Rao MV, Lam W, Green-Thompson ZW, Nixon RA, Saksida LM, Bussey TJ, O’Kane CJ, Rubinsztein DC (2015) Calpain inhibition mediates autophagy-dependent protection against polyglutamine toxicity. Cell Death Differ 22:433-444.
Miao Y, Dong LD, Chen J, Hu XC, Yang XL, Wang Z (2012) Involvement of calpain/p35-p25/Cdk5/NMDAR signaling pathway in glutamate-induced neurotoxicity in cultured rat retinal neurons. PLoS One 7:e42318.
Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, Menissier-de Murcia J, Susin SA (2007) Sequential activation of poly(ADP-ribose)polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol Cell Biol 27:4844-4862.
Mount MP, Zhang Y, Amini M, Callaghan S, Kulczycki J, Mao Z, Slack RS,Anisman H, Park DS (2013) Perturbation of transcription factor Nur77 expression mediated by myocyte enhancer factor 2D (MEF2D) regulates dopaminergic neuron loss in response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). J Biol Chem 288:14362-14371.
Muller GJ, Hasseldam H, Rasmussen RS, Johansen FF (2014) Dexamethasone enhances necrosis-like neuronal death in ischemic rat hippocampus involving mu-calpain activation. Exp Neurol 261:711-719.
Oh SH, Lim SC (2006) A rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N-acetylcysteine-mediated catalase upregulation. Toxicol Appl Pharm 212:212-223.
Ohsumi Y (2014) Historical landmarks of autophagy research. Cell Res 24:9-23.
Ono Y, Saido TC, Sorimachi H (2016) Calpain research for drug discovery:challenges and potential. Nat Rev Drug Discov 15:854-876.
Ozaki T, Ishiguro S, Hirano S, Baba A, Yamashita T, Tomita H, Nakazawa M (2013) Inhibitory peptide of mitochondrial mu-calpain protects against photoreceptor degeneration in rhodopsin transgenic S334ter and P23H rats.PLoS One 8:e71650.
Pandey AK, Shukla SC, Bhattacharya P, Patnaik R (2016) A possible therapeutic potential of quercetin through inhibition of mu-calpain in hypoxia induced neuronal injury: a molecular dynamics simulation study. Neural Regen Res 11:1247-1253.
Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in in flammation. Nature 517:311-320.
Prasad V, Kaestner V (2017) Nivolumab and pembrolizumab: Monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeable.Semin Oncol 44:132-135.
Rodriguez-Muela N, Hernandez-Pinto AM, Serrano-Puebla A, Garcia-Ledo L,Latorre SH, de la Rosa EJ, Boya P (2015) Lysosomal membrane permeabilization and autophagy blockade contribute to photoreceptor cell death in a mouse model of retinitis pigmentosa. Cell Death Differ 22:476-487.
Russo R, Berliocchi L, Adornetto A, Varano GP, Cavaliere F, Nucci C, Rotiroti D, Morrone LA, Bagetta G, Corasaniti MT (2011) Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis 2:e144.
Saatman KE, Bozyczko-Coyne D, Marcy V, Siman R, McIntosh TK (1996a)Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J Neuropath Exp Neur 55:850-860.
Saatman KE, Murai H, Bartus RT, Smith DH, Hayward NJ, Perri BR, McIntosh TK (1996b) Calpain inhibitor AK295 attenuates motor and cognitive de ficits following experimental brain injury in the rat. Proc Natl Acad Sci U S A 93:3428-3433.
Sacca E, Pizzutti N, Corazzin M, Lippe G, Piasentier E (2016) Assessment of calpain and caspase systems activities during ageing of two bovine muscles by degradation patterns of alphaII spectrin and PARP-1. Anim Sci J 87:462-466.
Sevrioukova IF (2011) Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid Redox Sign 14:2545-2579.
Shang L, Huang JF, Ding W, Chen S, Xue LX, Ma RF, Xiong K (2014) Calpain:a molecule to induce AIF-mediated necroptosis in RGC-5 following elevated hydrostatic pressure. Bmc Neurosci 15:63.
Shang L, Ding W, Li N, Liao L, Chen D, Huang J, Xiong K (2017) The effects and regulatory mechanism of RIP3 on RGC-5 necroptosis following elevated hydrostatic pressure. Acta Bioch Bioph Sin 49:128-137.
Singh R, Brewer MK, Mashburn CB, Lou D, Bondada V, Graham B, Geddes JW (2014) Calpain 5 is highly expressed in the central nervous system (CNS),carries dual nuclear localization signals, and is associated with nuclear promyelocytic leukemia protein bodies. J Biol Chem 289:19383-19394.
Song S, Tan J, Miao Y, Li M, Zhang Q (2017) Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J Cell Physiol 232:2977-2984.
Soong G, Chun J, Parker D, Prince A (2012) Staphylococcus aureus activation of caspase 1/calpain signaling mediates invasion through human keratinocytes. J Infect Dis 205:1571-1579.
Sosna J, Voigt S, Mathieu S, Lange A, Thon L, Davarnia P, Herdegen T,Linkermann A, Rittger A, Chan FK, Kabelitz D, Schutze S, Adam D (2014)TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol Life Sci 71:331-348.
Sun GZ, Gao FF, Zhao ZM, Sun H, Xu W, Wu LW, He YC (2016) Endoplasmic reticulum stress-induced apoptosis in the penumbra aggravates secondary damage in rats with traumatic brain injury. Neural Regen Res 11:1260-1266.
Suzuki K, Hata S, Kawabata Y, Sorimachi H (2004) Structure, activation, and biology of calpain. Diabetes 53 Suppl 1:S12-18.
Suzuki S, Murotomi K, Nakajima Y, Kawai K, Ohta K, Warita K, Miki T,Takeuchi Y (2014) Development of an artificial calcium-dependent transcription factor to detect sustained intracellular calcium elevation. Acs Synth Biol 3:717-722.
Tan Y, Wu C, De Veyra T, Greer PA (2006) Ubiquitous calpains promote both apoptosis and survival signals in response to different cell death stimuli. J Biol Chem 281:17689-17698.
Thornton C, Leaw B, Mallard C, Nair S, Jinnai M, Hagberg H (2017) Cell death in the developing brain after hypoxia-ischemia. Front Cell Neurosci 11:248.
Van Wijk SJ, Hageman GJ (2005) Poly(ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusion. Free Radical Bio Med 39:81-90.
Villalpando Rodriguez GE, Torriglia A (2013) Calpain 1 induce lysosomal permeabilization by cleavage of lysosomal associated membrane protein 2.Biochim Biophys Acta 1833:2244-2253.
Wang C, Shi D, Song X, Chen Y, Wang L, Zhang X (2016a) Calpain inhibitor attenuates ER stress-induced apoptosis in injured spinal cord after bone mesenchymal stem cells transplantation. Neurochem Int 97:15-25.
Wang S, Liao L, Wang M, Zhou H, Huang Y, Wang Z, Chen D, Ji D, Xia X,Wang Y, Liu F, Huang J, Xiong K (2018) Pin1 promotes regulated necrosis induced by glutamate in rat retinal neurons via CAST/Calpain2 pathway.Front Cell Neurosci doi: 10.3389/fncel.2017.00425.
Wang Y, Kim NS, Li X, Greer PA, Koehler RC, Dawson VL, Dawson TM (2009)Calpain activation is not required for AIF translocation in PARP-1-dependent cell death (parthanatos). J Neurochem 110:687-696.
Wang Y, Zyskind JW, Colacurcio DJ, Lindl KA, Ting JH, Grigoriev G,Jordan-Sciutto KL (2012) Differential roles for caspase-mediated and calpain-mediated cell death in 1- and 3-week-old rat cortical cultures. Neuroreport 23:1052-1058.
Wang Y, Lopez D, Davey PG, Cameron DJ, Nguyen K, Tran J, Marquez E, Liu Y, Bi X, Baudry M (2016b) Calpain-1 and calpain-2 play opposite roles in retinal ganglion cell degeneration induced by retinal ischemia/reperfusion injury. Neurobiol Dis 93:121-128.
Wang Z, Li HP, He XJ, Ji G, Zhang J, Nian YW, Zhang K (2016c) Expression of apoptosis-related protein in motor neurons of anterior horn of the spinal cord after acute cauda equina compression. Zhongguo Zuzhi Gongcheng Yanjiu 20:671-676.
Watchon M, Yuan KC, Mackovski N, Svahn AJ, Cole NJ, Goldsbury C, Rinkwitz S, Becker TS, Nicholson GA, Laird AS (2017) Calpain inhibition is protective in machado-joseph disease zebra fish due to induction of autophagy. J Neurosci 37:7782-7794.
Weber JJ, Golla M, Guaitoli G, Wanichawan P, Hayer SN, Hauser S, Krahl AC,Nagel M, Samer S, Aronica E, Carlson CR, Schols L, Riess O, Gloeckner CJ,Nguyen HP, Hubener-Schmid J (2017) A combinatorial approach to identify calpain cleavage sites in the Machado-Joseph disease protein ataxin-3.Brain doi: 10.1093/brain/awx039.
Williams A, Sarkar S, Cuddon P, TtofiEK, Saiki S, Siddiqi FH, Jahreiss L,Fleming A, Pask D, Goldsmith P, O’Kane CJ, Floto RA, Rubinsztein DC(2008) Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol 4:295-305.
Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter DA, Lynch DR (2007)Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem 282:20075-20087.
Wu XG, Qiu ZF, Meng J, Zu BX, Li MM, Miao H (2016) Effects of Buyanghuanwu decoction on the protein expression of PI3K, Akt, Bcl-2 and BAX in brain tissue of a rat model of cerebral hemorrhage. Zhongguo Zuzhi Gongcheng Yanjiu 20:5933-5938.
Xiong K, Liao H, Long L, Ding Y, Huang J, Yan J (2016) Necroptosis contributes to methamphetamine-induced cytotoxicity in rat cortical neurons.Toxicol Vitro 35:163-168.
Yamada KH, Kozlowski DA, Seidl SE, Lance S, Wieschhaus AJ, Sundivakkam P, Tiruppathi C, Chishti I, Herman IM, Kuchay SM, Chishti AH (2012) Targeted gene inactivation of calpain-1 suppresses cortical degeneration due to traumatic brain injury and neuronal apoptosis induced by oxidative stress. J Biol Chem 287:13182-13193.
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12:814-822.
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47-59.
Youse fiS, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU (2006) Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 8:1124-1132.
Zhang JY, Peng C, Shi H, Wang S, Wang Q, Wang JZ (2009) Inhibition of autophagy causes tau proteolysis by activating calpain in rat brain. J Alzheimers Dis 16:39-47.
Zhang Q, Xie H, Ji Z, He R, Xu M, He Y, Huang J, Pan S, Hu Y (2016) Cdk5/p25 specific inhibitory peptide TFP5 rescues the loss of dopaminergic neurons in a sub-acute MPTP induced PD mouse model. Neurosci Lett 632:1-7.
Zhou F, Yang Y, Xing D (2011) Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J 278:403-413.
- 中國神經(jīng)再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Optic radiation injury in patients with aneurismal subarachnoid hemorrhage: a preliminary diffusion tensor imaging report
- The ROCK pathway inhibitor Y-27632 mitigates hypoxia and oxidative stress-induced injury to retinal Müller cells
- Combined acupuncture and HuangDiSan treatment affects behavior and synaptophysin levels in the hippocampus of senescence-accelerated mouse prone 8 after neural stem cell transplantation
- Endoplasmic reticulum stress transducer old astrocyte speci fically induced substance contributes to astrogliosis after spinal cord injury
- DTI and pathological changes in a rabbit model of radiation injury to the spinal cord after 125I radioactive seed implantation

