In vitro neuroprotective effects of ciliary neurotrophic factor on dorsal root ganglion neurons with glutamate-induced neurotoxicity
Shu-yun wen, Ai-min Li, Kuan-qing Mi, Rui-zheng wang, Jao Li, Jua-xiang Liu, Yi Xing
1 Department of Neurosurgery, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong Province, China
2 Department of Rheumatology, Qilu Hospital, Shandong University, Jinan, Shandong Province, China
3 Department of Rheumatology, Qingdao Fifth People’s Hospital, Qingdao, Shandong Province, China
4 Department of Neurosurgery, Jinan Fifth People’s Hospital, Jinan, Shandong Province, China
5 Department of Orthopedics, Qilu Hospital, Shandong University, Jinan, Shandong Province, China
How to cite this article: Wen SY, Li AM, Mi KQ, Wang RZ, Li H, Liu HX, Xing Y (2017) In vitro neuroprotective effects of ciliary neurotrophic factor on dorsal root ganglion neurons with glutamate-induced neurotoxicity. Neural Regen Res 12(10):1716-1723.
Funding: is work was supported by the Natural Science Foundation of Shandong Province of China, No. ZR2014HQ065; a grant from the Medical Science and Technology Development Project of Shandong Province of China, No. 2015WS0445.
In vitro neuroprotective effects of ciliary neurotrophic factor on dorsal root ganglion neurons with glutamate-induced neurotoxicity
Shu-yun wen1,2, Ai-min Li3, Kuan-qing Mi4, Rui-zheng wang4, Jao Li5, Jua-xiang Liu2, Yi Xing1,*
1 Department of Neurosurgery, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong Province, China
2 Department of Rheumatology, Qilu Hospital, Shandong University, Jinan, Shandong Province, China
3 Department of Rheumatology, Qingdao Fifth People’s Hospital, Qingdao, Shandong Province, China
4 Department of Neurosurgery, Jinan Fifth People’s Hospital, Jinan, Shandong Province, China
5 Department of Orthopedics, Qilu Hospital, Shandong University, Jinan, Shandong Province, China
How to cite this article: Wen SY, Li AM, Mi KQ, Wang RZ, Li H, Liu HX, Xing Y (2017) In vitro neuroprotective effects of ciliary neurotrophic factor on dorsal root ganglion neurons with glutamate-induced neurotoxicity. Neural Regen Res 12(10):1716-1723.
Ciliary neurotrophic factor has neuroprotective effects mediated through signal transducer and Janus kinase (JAK) 2/activator of transcription 3 (STAT3) and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways. Whether ciliary neurotrophic factor is neuroprotective for glutamate-induced excitotoxicity of dorsal root ganglion neurons is poorly understood. In the present study, the in vitro neuroprotective effects of ciliary neurotrophic factor against glutamate-induced excitotoxicity were determined in a primary culture of dorsal root ganglion neurons from Wistar rat embryos at embryonic day 15. Whether the JAK2/STAT3 and PI3K/Akt signaling pathways were related to the protective effects of ciliary neurotrophic factor was also determined. Glutamate exposure inhibited neurite outgrowth, cell viability, and growth-associated protein 43 expression and promoted apoptotic neuronal cell death, all of which were reversed by the administration of exogenous ciliary neurotrophic factor. Additionally, preincubation with either JAK2 inhibitor AG490 or PI3K inhibitor LY294002 blocked the neuroprotective effect of ciliary neurotrophic factor.ese data indicate that the two pathways JAK2/STAT3 and PI3K/Akt play major roles in mediating the in vitro neuroprotective effects of ciliary neurotrophic factor on dorsal root ganglion neurons with glutamate-induced neurotoxicity.
nerve regeneration; ciliary neurotrophic factor; JAK2/STAT3; PI3K/Akt; glutamate; neuron; excitotoxicity; neuroprotection;growth-associated protein 43; neurite outgrowth; dorsal root ganglion; neural regeneration
Introduction
Ciliary neurotrophic factor (CNTF), belonging to the interleukin-6 cytokine family, is widely known as a neurocytokine that exerts multiple effects on a broad range of neurons in the central and peripheral nervous systems (Siegel et al.,2000; Sango et al., 2008; Lee et al., 2017).e upregulation of CNTF has a protective effect aer spinal cord injury (Chen et al., 2015; Feng et al., 2017; Hodgetts and Harvey, 2017).CNTF expressed in local spinal nerves is a likely trigger of corticospinal tract axon sprouting (Jin et al., 2015). As a neurotrophic factor, CNTF promotes the long-distance regrowth of severed optic nerve fibers aer intracranial injury(Vieira et al., 2009; Lee et al., 2016; LeVaillant et al., 2016;Yin et al., 2016; Beach et al., 2017; Cen et al., 2017; Jindal et al., 2017; Zhou et al., 2017). Recently, CNTF has gained interest because of its key role in the regeneration of injured peripheral nerves (Barbon et al., 2016; Lee et al., 2017).CNTF is critically important for promoting the survival of postmitotic neurons and the genesis and differentiation of neurons in the immature dorsal root ganglion (DRG) by stimulating neurons to release mitogenic factors (Hapner et al., 2006).ese are the key functions of CNTF to maintain the normal development of different subpopulations of neurons in DRG. Both small and large subpopulations of DRG neurons are supported by neurotrophic actions derived from CNTF (Sango et al., 2008). A previous in vitro study reported abundant CNTF mRNA and protein expressed in neurons in a dissociated DRG cell culture. CNTF was also expressed in DRG neuronal cell bodies and in regenerating neurites,suggesting that the synthesis site is at the neuronal cell body and that peripheral CNTF in neurites is transported from the neuronal cell bodies over time during cell culture (Sango et al., 2007).e upregulation of CNTF in DRG suggested it had a restorative effect aer sciatic nerve crush injury in mice (Gan et al., 2014). Chemical acellular allogeneic nerve gras combined with CNTF repaired sciatic nerve defects in rats. Both in vitro and in vivo experiments revealed that the induction of DRG axonal regeneration by CNTF was mediated by the upregulation of a target gene, collapsin response-mediator protein 4 (Jang et al., 2010; Selvaraj and Sendtner, 2013). CNTF was reported to have neuroprotective effects on DRG neurons under various conditions(Hapner et al., 2006; Liu et al., 2014). Several recent studies of CNTF targeting on developing (immature) and adult(mature) DRG neurons confirmed that CNTF is a critical neurotrophic factor that maintains normal DRG development and DRG neuronal regeneration after injury (Zhou et al., 2008; Gu et al., 2010; Kammouni et al., 2012; Saleh et al., 2013; Chowdhury et al., 2014).
Glutamate (Glu) is an excitatory neurotransmitter that plays a major role in the nervous system.e dysregulation of Glu may induce seizures (Takanashi et al., 2015).e Glu receptors are also related to ischemia induced neurotoxicity(Brassai et al., 2015). Glu is also shown to be important for primary afferent neurons (Brumovsky et al., 2011). Glu also causes excitatory neurotoxicity of cultured DRG neurons (Liu et al., 2011, 2012; Li et al., 2013a, b). Neuronal cell damage or cell death induced by excitatory excitotoxicity is caused by high permeability to calcium via activation of NMDA receptor subtypes (Del Río et al., 2008). Furthermore, CNTF had a neuroprotective role after neuronal injury through the activation of PI3K/Akt signaling and signal transducer and activator of transcription 3 (STAT3) signaling in vitro(Leibinger et al., 2014; Liu et al., 2014; Gu et al., 2016). However, whether the neurotrophic factor CNTF is neuroprotective for developing DRG neurons from later gestational age with Glu challenge in vitro is unclear. Based on the above research, we hypothesized that: (1) CNTF has neuroprotective effects on DRG neurons with Glu-induced excitotoxicity;and (2) the neuroprotective effects of CNTF are mediated through the activation of JAK2/STAT3 and PI3K/Akt signaling pathways.is is the first report to investigate the effects of CNTF and its downstream signaling pathways on primary sensory neuronal protection by establishing an in vitro model of dissociated DRG neurons.e data from this experimental research may offer novel therapeutic options on relieving Glu-induced excitotoxicity by the administration of CNTF under distinct neuropathological conditions.
Materials and Methods
DRG culture preparations
All animals used in this study were from the Experimental Animal Center of Shandong University of China (animal license No. SCXK (Lu) 20130009). The study protocol was approved by the Ethics Committee for Animal Experimentation of the Shandong University (approval No.201402260001). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23,revised 1986).
Male or female Wistar rat embryos at embryonic day 15 were dissected for DRG neuronal culture. To obtain a suspension of DRG cells, DRG explants were processed for digestion (0.25% trypsin; Sigma, St. Louis, MO, USA), centrifugation (1,000 r/min, 5 minutes), trituration, and then filtered with a 130-μm filter. Before plating, the number of cells in the cell suspension was counted to determine the cell density. Dissociated DRG cells were plated at a density of 5 × 105cells/mL for PCR or western blot assay and 1 ×105cells/well for fluorescence labeling in 24-well plates.DRG cells were plated at 5 × 104cells/well in a volume of 0.1 mL in 96-well plates for cell viability measurement. After plating, all cells were maintained in Dulbecco’s Modified Eagle’s Medium/F-12 (HyClone Laboratories, Logan, UT,USA), containing 5% fetal bovine serum, 2% B-27 supplement (Gibco, Grand Island, NY, USA), and L-glutamine (0.1 mg/mL, Sigma), and incubated at 37°C in a 5% CO2incubator for 24 hours and then maintained in culture medium containing cytosine arabinoside(5 μg/mL; Solarbio Life Sciences, Beijing, China) for an additional 24 hours to inhibit the growth of non-neuronal cells.en, the culture medium was removed and DRG cells were exposed to various agents for an additional 24 hours before final examination.
Incubation of DRG neurons under different experimental conditions
At 48 hours of culture, dissociated cultured DRG cells were treated for another 24 hours. The incubation paradigm in this study was as follows: (1) Glu group: Glu 200 μM incubation for 24 hours; (2) Glu + CNTF group: Glu 200 μM plus CNTF 25 ng/mL incubation for 24 hours; (3) Glu + CNTF+ AG490 group: JAK2 inhibitor AG490 (10 μM) incubation for 30 minutes followed by treatment with Glu 200 μM plus CNTF 25 ng/mL for 24 hours; (4) Glu + CNTF + LY294002 group: PI3K inhibitor LY294002 (20 μM) incubation for 30 minutes followed by treatment with Glu 200 μM plus CNTF 25 ng/mL for 24 hours; and (5) Control group: neurons were cultured in culture medium alone without any other treat-ment.e vehicle solution used in all groups of this in vitro experiment was normal culture medium, which did not interfere the observation results. Five samples were examined in each group (n = 5).e concentrations and time point of the inhibitor administration (AG490 and LY294002) were based on previous studies (Li et al., 2013b; Liu et al., 2014)and slightly modified according to our preliminary experiment. All cultures mentioned above were incubated at 37°C in a humidified atmosphere of 5% CO2-air.
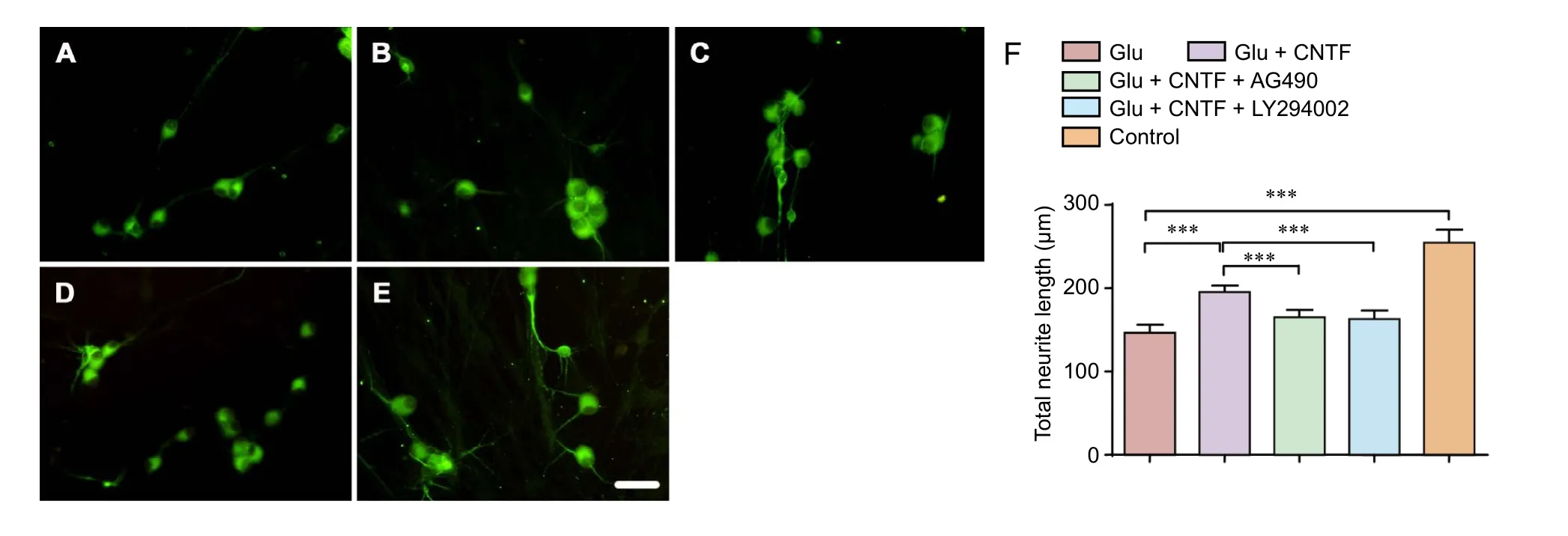
Figure 1 Effects of CNTF on the growth of dorsal root ganglion neurons induced by Glu.
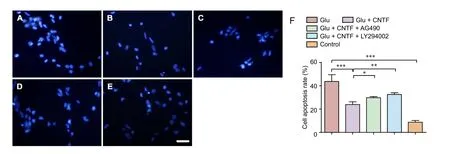
Figure 3 Effects of CNTF on cell apoptosis in dorsal root ganglion neurons induced by Glu.
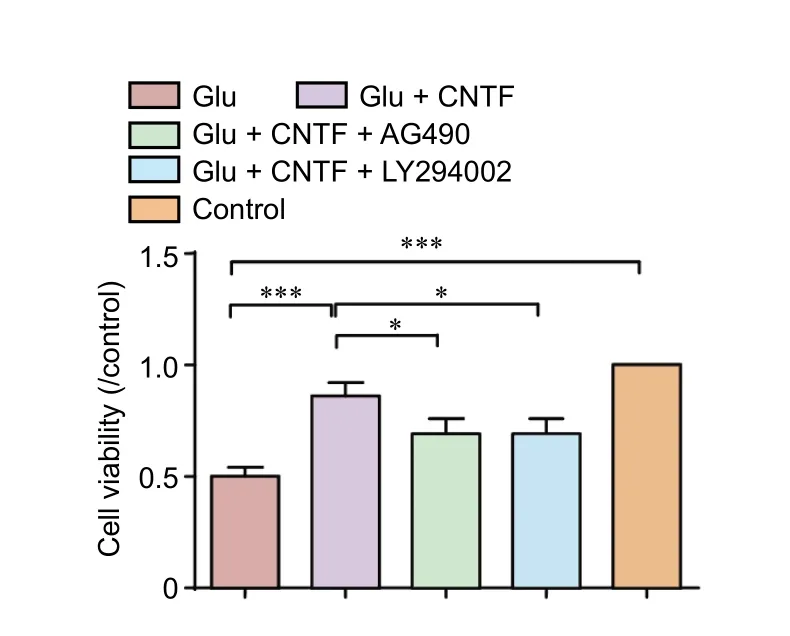
Figure 2 Effects of CNTF on cell viability of dorsal root ganglion neurons induced by Glu.
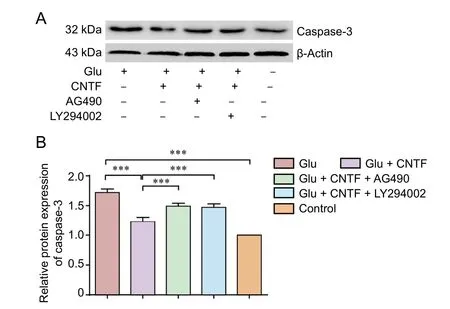
Figure 4 Effects of CNTF on caspase-3 level in dorsal root ganglion neurons induced by Glu.
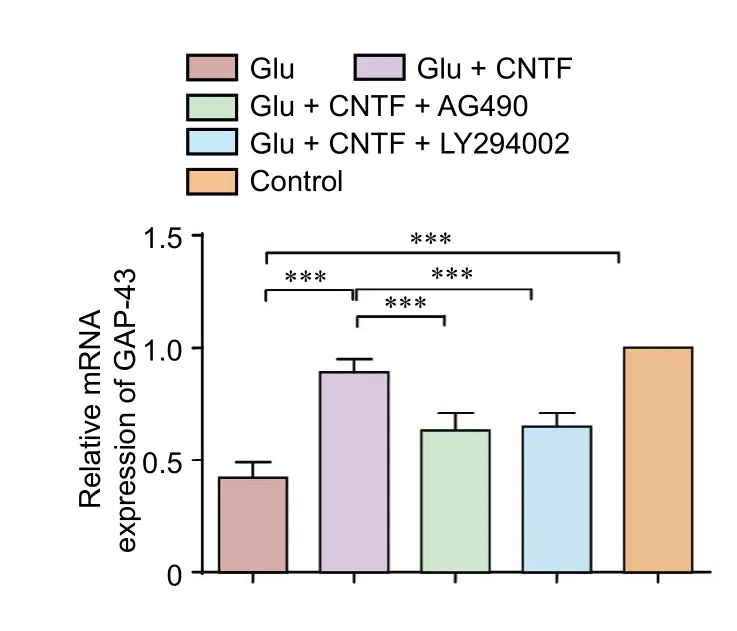
Figure 5 Effects of CNTF on GAP-43 mRNA levels in dorsal root ganglion neurons induced by Glu.
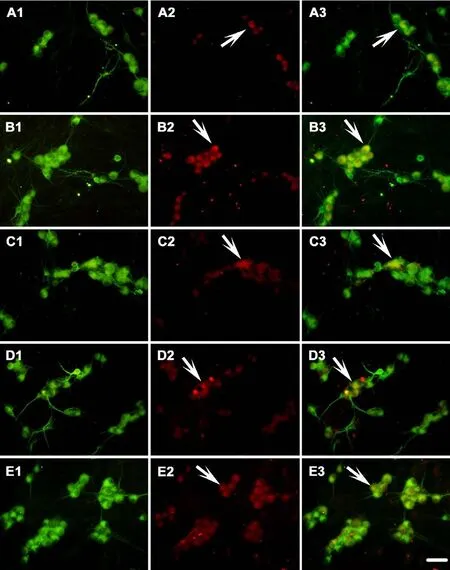

Figure 6 Effects of CNTF on the GAP-43 immunoreactivity of dorsal root ganglion neurons induced by Glu.
Neurite length measurement
Assessment of neuronal cell viability
Measurement of DRG neuron apoptosis by Hoechst 33342 staining
After incubation under different experimental conditions for 24 hours, neuronal apoptosis was evaluated by staining with Hoechst 33342, which directly shows morphological alterations of the nucleus in each neuron. Nuclear DNA was labeled by Hoechst 33342 (Sigma). Aer termination of the culture procedure at the designed incubation time point,neurons were grown on the surface of coverslips fixed with 4% paraformaldehyde for 10 minutes.en, the neurons were stained with Hoechst 33342 (5 μg/mL) for 5 minutes in the dark to preserve fluorescence intensity. Apoptotic neurons showed shrunken or condensed nuclei after Hoechst 33342 staining. Neurons with shrunken or condensed nuclei in five visual fields (400×) in the central part of each coverslip were counted as apoptotic neurons in each sample. Simultaneously,all neurons in the same five visual fields were also counted as the total number of neurons.e ratio of apoptotic neurons to total number of neurons was determined.
Measurement of DRG neuron apoptosis by caspase-3 expression
After incubation under different experimental conditions for 24 hours, neuron apoptosis was analyzed by detecting caspase-3 levels by western blot assay.e western blot protocol for caspase-3 was performed using rabbit anti-caspase-3 polyclonal IgG (1:1,000; Cell Signaling Technology, Danvers,MA, USA) or mouse anti-β-actin monoclonal IgG (1:1,000;Santa Cruz Biotechnology, Santa Cruz, CA, USA) as the primary antibodies.e secondary antibodies were goat anti-rabbit IgG-horseradish peroxidase (HRP) (1:6,000; Beijing Sequoia Jinqiao Biological Technology Co., Ltd., Beijing,China) or goat anti-mouse IgG-HRP (1:3,000; Beijing Dingguochangsheng Biotechnology Co., Ltd., Beijing, China).e gray-scale values of immunoreactive bands were obtained by ImageJ (National Institutes of Health).e value of the target protein was compared with the internal control protein β-actin. Each experimental group was compared relative to the control group value (1.00) to obtain a ratio.
Determination of growth-associated protein 43 (GAP-43)mRNA levels with real-time polymerase chain reaction(PCR)
After incubation under different experimental conditions for 24 hours, real-time PCR was performed to detect GAP-43 mRNA expression, using GAPDH mRNA expression as an internal control. Total RNA isolation was performed with TRIzol (Takara Biotechnology, Dalian, China). Synthesis of cDNA was performed using a cDNA synthesis kit (ermo Scientific Molecular Biology, Lithuania, EU, USA) following the manufacturer’s instructions. Synthetic oligonucleotide primer sequences for GAP-43 and GAPDH are shown inTable 1. A comparative cycle of the threshold fluorescence (Ct)method was used (Thermo Scientific Molecular Biology).The relative transcript amount of the target gene was normalized to that of GAPDH using the 2?ΔΔCtmethod based on a previous study (Pfaffl, 2001).e fold change of each target gene was calculated.e final results of real-time PCR were expressed as a ratio of fold change of target mRNA of that to the control group.
Double fluorescent labeling to assess GAP-43 and MAP2 expression
After incubation under different experimental conditions for 24 hours, double fluorescence labeling for GAP-43 and MAP2 expression was performed using rabbit polyclonal anti-GAP-43 (1:500; Abcam, Cambridge, MA, USA) or mousemonoclonal anti-MAP2 (1:400; Abcam) as the primary antibodies.e secondary antibodies were goat anti-rabbit conjugated to Cy3 (1:200; Abcam) or goat anti-mouse conjugated to Cy2 (1:200; Abcam). All procedures were performed in the dark to preserve fluorescence. Before incubation with the primary antibody, the cells were processed for fixation, non-specific site blocking, and permeabilization.ree washes with 0.1 M phosphate buffered saline were carried out between each step before covering with a coverslip. Slide observation and image capture were performed under a fluorescent microscope (Olympus BX63, Olympus Corporation, Tokyo, Japan)with a digital photo processing system (cellSens Dimension,version 1.6, Olympus Corporation, Tokyo, Japan).
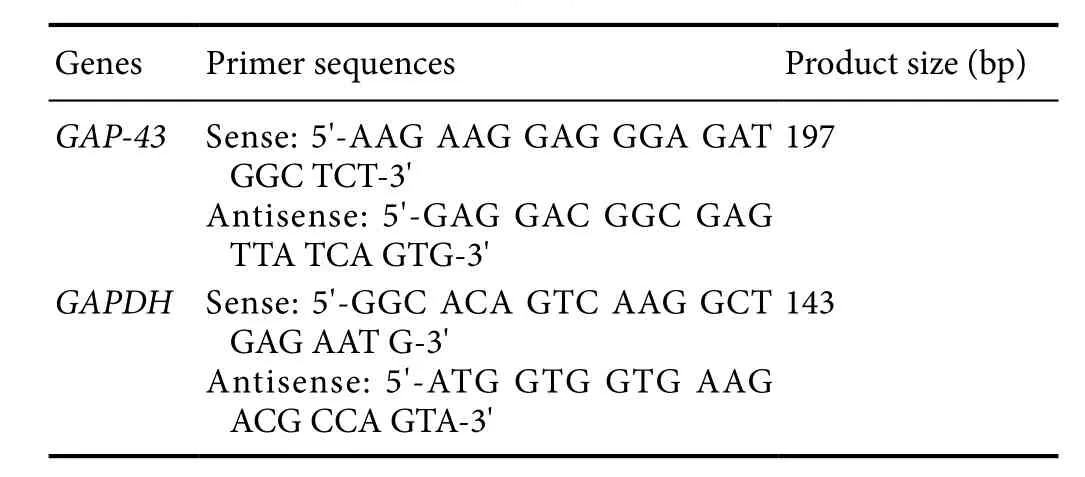
Table 1 Primer sequences of target genes
Quantification of GAP-43-immunoreactive (IR) neuronsGAP-43-IR neurons were counted in a 200× visual field with red fluorescence. Five adjacent, but not overlapping, visual fields in the center of the coverslip were selected for GAP-43-IR neuronal counting. The same five visual fields with green fluorescence were used for MAP2-IR neuronal counting. Because each neuron expressed MAP2, the amount of MAP2-IR neuron was used as the total amount of DRG neurons.e proportion of GAP-43-IR neurons was calculated relative to the total number of neurons.
Statistical analysis
Quantitative data were expressed as the mean ± SD. Data were analyzed using SPSS 19.0 software (SPSS, Chicago,IL, USA). All quantitative data were verified by normality testing. All data were normally distributed and were analyzed by analysis of variance followed by Dunnett’s T3 test(heterogeneity of variance) or Student-Newman-Keuls test(homogeneity of variance). A P value < 0.05 was considered statistically significant.
Results
Neurite outgrowth
Neuronal cell viability
The cell viability under different experimental conditions was monitored using a WST-1 kit after termination of the culture procedure at the designated incubation time point.e cell viability was decreased significantly aer treatment with Glu (P = 0.000). Treatment with exogenous CNTF partially reversed the Glu-induced decrease in cell viability (P =0.000). Preincubation with the JAK2 inhibitor AG490 (P =0.031) or PI3K inhibitor LY294002 (P = 0.031) blocked the promoting effects of CNTF on cell viability (Figure 2).
Apoptosis of DRG neurons
The apoptotic rate of neuronal cells was determined by Hoechst 33342 staining aer termination of the culture procedure at the designated incubation time point. Glu exposure increased the neuronal cell apoptotic rate (P = 0.001),which was partially inhibited by CNTF treatment (P = 0.005).Preincubation with the JAK2 inhibitor AG490 (P = 0.023) or PI3K inhibitor LY294002 (P = 0.002) blocked the inhibitory effects of CNTF on apoptosis (Figure 3).
Caspase-3 levels in DRG neurons
To investigate the apoptosis of DRG neurons under each experimental condition, caspase-3 levels in DRG neurons were quantified by western blot assay. Glu exposure increased the caspase-3 levels in DRG neurons (P < 0.001), which was partially decreased by CNTF treatment (P < 0.001). Preincubation with the JAK2 inhibitor AG490 (P < 0.001) or PI3K inhibitor LY294002 (P < 0.001) blocked the inhibitory effects of CNTF on caspase-3 expression (Figure 4).
GAP-43 mRNA expression in DRG neurons
GAP-43 expression in DRG neurons
To determine the GAP-43 expression in situ in DRG neurons, double fluorescence labeling of GAP-43 and MAP2 was performed to quantify the proportion of GAP-43 positive neurons under various experimental conditions. Glu exposure decreased the proportion of GAP-43-IR neurons (P< 0.001). The application of exogenous CNTF in DRG cultures increased the proportion of GAP-43-IR neurons, which was decreased by Glu exposure (P < 0.001). Preincubation with the JAK2 inhibitor AG490 (P < 0.01) or PI3K inhibitor LY294002 (P < 0.01) blocked the increased proportion of GAP-43-IR neurons mediated by CNTF.ese results suggest that CNTF partially rescues the neuronal regeneration capacity inhibited by Glu exposure and the effects of CNTF on the increased proportion of GAP-43-IR neurons by activating the JAK2/STAT3 and PI3K/Akt signaling pathways (Figure 6).
Discussion
CNTF is a multifunctional neurocytokine and is involved in the genesis, differentiation, and survival of various kinds of neurons, including primary sensory neurons in the central and peripheral nervous systems. CNTF is critical for promoting the survival of postmitotic neurons in DRG. CNTF also regulates earlier events in the developing DRG, related to the mitogenesis of DRG progenitor cells and the differentiation of immature DRG neurons.e JAK2/STAT3 and PI3K/Akt signaling pathways are involved in neurogenesis, proliferation,differentiation, and neuroprotection under various conditions.We determined the neuroprotective effect of CNTF against excitotoxicity in DRG neurons by administering CNTF to dissociated DRG cultures with Glu challenge.e present study is the first to investigate the neuroprotective effects of CNTF on primary sensory DRG neurons with Glu-induced neurotoxicity. CNTF administration partially reversed Glu-induced neurotoxicity by promoting GAP-43 expression, increasing cell viability, enhancing neurite outgrowth, and inhibiting apoptotic neuronal cell death. Furthermore, the inhibition of JAK2/STAT3 signaling by preincubation with AG490 or PI3K/Akt signaling by preincubation with LY294002 blocked the neuroprotective actions of CNTF. These data imply the capacity of CNTF to relieve excitotoxicity.
Glu killed oligodendrocytes and neurons in the rat spinal cord. It was proposed that the activation of apoptosis is inversely correlated with the concentration of intracellular calcium in immature DRG neurons in dissociated cell cultures.Glu exposure might cause neurite retraction by inhibiting GAP-43 expression in dissociated DRG cell cultures (Li et al.,2013b). In our present study, the inhibitory actions of Glu on neurite outgrowth and cell viability and the promotion of apoptosis were partially reversed by CNTF in DRG cultures,suggesting the neuroprotective effects of CNTF on Glu-induced neurotoxicity. To elucidate the mechanisms of the neuroprotective effects of CNTF, inhibitors that block JAK2 or PI3K signaling were used in DRG neuronal cultures.e JAK2 inhibitor AG490 or PI3K inhibitor LY294002 blocked the protective effect of CNTF on neurons.ese data imply that the two signaling pathways are involved in the neuroprotective effects of CNTF in distinct DRG cell cultures.
To explore further the relationship between neurite elongation and nerve regeneration marker GAP-43, GAP-43 mRNA levels and GAP-43 expression in situ in DRG neurons were also evaluated. GAP-43 was used as a marker or indicator to assess regeneration, active axonal sprouting, and neuronal survival under in vivo (Gravel et al., 2011) and in vitro conditions (Anand et al., 2008) and neurite elongation was strongly associated with GAP-43 levels in DRG neurons in vitro(Tsai et al., 2007; Li et al., 2013b).e results of the present study showed CNTF increased the proportion of GAP-43-IR neurons. This suggests that the promoting effects of CNTF on neuronal regeneration might be mediated by initiating the neurite regeneration capacity inhibited by Glu exposure.Because Glu exposure significantly decreased the proportion of GAP-43-IR neurons, Glu-induced excitotoxicity might destroy the regeneration ability of neurons sensitive to Glu stimulation.erefore, exogenous CNTF administration may rescue the regeneration ability of excitotoxic neurons.
In conclusion, CNTF relieved the Glu-induced neurotoxicity of DRG neurons by inhibiting apoptotic neuronal cell death and initiating GAP-43 expression to increase neuronal cell viability, promoting neurite outgrowth, and finally improving neuronal living status.e JAK2/STAT3 and PI3K/Akt signaling pathways play major roles in mediating the neuroprotective effects of CNTF on DRG neurons undergoing neurotoxicity caused by excessive Glu challenge. These data imply that CNTF might be a novel therapeutic option to relieve Glu-induced excitotoxicity under distinct neuropathological conditions.
Author contributions:SYW and YX designed this study. SYW and HL performed experiments. AML, KQM, RZW, and HL analyzed data.HXL and YX wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:e study protocol was approved by the Ethical Committee for Animal Experimentation of the Shandong University (approval No. 201402260001). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Anand U, Otto WR, Bountra C, Chessell I, Sinisi M, Birch R, Anand P(2008) Cytosine arabinoside affects the heat and capsaicin receptor TRPV1 localisation and sensitivity in human sensory neurons. J Neurooncol 89:1-7.
Barbon S, Stocco E, Negro A, Dalzoppo D, Borgio L, Rajendran S, Grandi F, Porzionato A, Macchi V, De Caro R, Parnigotto PP, Grandi C (2016)In vitro assessment of TAT- Ciliary Neurotrophic Factor therapeutic potential for peripheral nerve regeneration. Toxicol Appl Pharmacol 309:121-128.
Beach KM, Wang J, Otteson DC (2017) Regulation of stem cell properties of müller glia by JAK/STAT and MAPK signaling in the mammalian retina. Stem Cells Int doi: 10.1155/2017/1610691.
Brassai A, Suvanjeiev RG, Bán EG, Lakatos M (2015) Role of synaptic and nonsynaptic glutamate receptors in ischaemia induced neurotoxicity. Brain Res Bull 112:1-6.
Brumovsky PR, Seroogy KB, Lundgren KH, Watanabe M, H?kfelt T,Gebhart GF (2011) Some lumbar sympathetic neurons develop a glutamatergic phenotype after peripheral axotomy with a note on VGLUT2-positive perineuronal baskets. Exp Neurol 230: 258-272.
Cen LP, Liang JJ, Chen JH, Harvey AR, Ng TK, Zhang M, Pang CP, Cui Q, Fan YM (2017) AAV-mediated transfer of RhoA shRNA and CNTF promotes retinal ganglion cell survival and axon regeneration. Neuroscience doi: 10.1016/j.neuroscience.2016.12.027.
Chen XB, Yuan H, Wang FJ, Tan ZX, Liu H, Chen N (2015) Protective role of selenium-enriched supplement on spinal cord injury through the up-regulation of CNTF and CNTF-Ralpha. Eur Rev Med Pharmacol Sci 19:4434-4442.
Chowdhury SR, Saleh A, Akude E, Smith DR, Morrow D, Tessler L, Calcutt NA, Fernyhough P (2014) Ciliary neurotrophic factor reverses aberrant mitochondrial bioenergetics through the JAK/STAT pathway in cultured sensory neurons derived from streptozotocin-induced diabetic rodents. Cell Mol Neurobiol 34:643-649.
Del Río P, Montiel T, Massieu L (2008) Contribution of NMDA and non-NMDA receptors to in vivo glutamate-induced calpain activation in the rat striatum. Relation to neuronal damage. Neurochem Res 33:1475-1483.
Denny JB (2006) Molecular mechanisms, biological actions, and neuropharmacology of the growth-associated protein GAP-43. Curr Neuropharmacol 4:293-304.
Feng GY, Liu J, Wang YC, Wang ZY, Hu Y, Xia QJ, Xu Y, Shang FF, Chen MR, Wang F, Zhou X, Wang TH (2017) Effects of alpha-synuclein on primary spinal cord neurons associated with apoptosis and cntf expression. Cell Mol Neurobiol doi: 10.1007/s10571-016-0420-x.
Gravel M, Weng YC, Kriz J (2011) Model system for live imaging of neuronal responses to injury and repair. Mol Imaging 10:434-445.
Gu Y, Wang J, Ding F, Hu N, Wang Y, Gu X (2010) Neurotrophic actions of bone marrow stromal cells on primary culture of dorsal root ganglion tissues and neurons. J Mol Neurosci 40:332-341.
Gu YL, Gao GQ, Ma N, Ye LL, Zhang LW, Gao X, Zhang ZB (2016)CNTF protects neurons from hypoxic injury through the activation of STAT3pTyr705. Int J Mol Med 38:1915-1921.
Hodgetts SI, Harvey AR (2017) Neurotrophic factors used to treat spinal cord injury. Vitam Horm doi: 10.1016/bs.vh.2016.11.007.
Jang SY, Shin YK, Jung J, Lee SH, Seo SY, Suh DJ, Park HT (2010) Injury-induced CRMP4 expression in adult sensory neurons; a possible target gene for ciliary neurotrophic factor. Neurosci Lett 485:37-42.
Jin D, Liu Y, Sun F, Wang X, Liu X, He Z (2015) Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat Commun 6:8074.
Jindal N, Banik A, Prabhakar S, Vaiphie K, Anand A (2017) Alteration of neurotrophic factors aer transplantation of bone marrow derived linve stem cell in NMDA-induced mouse model of retinal degeneration.J Cell Biochem doi: 10.1002/jcb.25827.
Kammouni W, Hasan L, Saleh A, Wood H, Fernyhough P, Jackson AC(2012) Role of nuclear factor-κB in oxidative stress associated with rabies virus infection of adult rat dorsal root ganglion neurons. J Virol 86:8139-8146.
Lee N, Rydyznski CE, Rasch MS, Trinh DS, MacLennan AJ (2017) Adult ciliary neurotrophic factor receptors help maintain facial motor neuron choline acetyltransferase expression in vivo following nerve crush.J Comp Neurol doi: 10.1002/cne.24126.
Lee N, Serbinski CR, Braunlin MR, Rasch MS, Rydyznski CE, MacLennan AJ (2016) Muscle and motor neuron ciliary neurotrophic factor receptor α together maintain adult motor neuron axons in vivo. Eur J Neurosci 44:3023-3034.
Leibinger M, Andreadaki A, Diekmann H, Fischer D (2013) Neuronal STAT3 activation is essential for CNTF- and inflammatory stimulation-induced CNS axon regeneration. Cell Death Dis 4:e805.
LeVaillant CJ, Sharma A, Muhling J, Wheeler LP, Cozens GS, Hellstr?m M, Rodger J, Harvey AR (2016) Significant changes in endogenous retinal gene expression assessed 1 year aer a single intraocular injection of AAV-CNTF or AAV-BDNF. Moler Methods Clin Dev 3:16078.
Li H, Dong H, Li J, Liu H, Liu Z, Li Z (2013a) Neuroprotective effect of insulin-like growth factor-1: Effects on tyrosine kinase receptor (Trk)expression in dorsal root ganglion neurons with glutamate-induced excitotoxicity in vitro. Brain Res Bull 97C:86-95.
Li Y, Li H, Liu G, Liu Z (2013b) Effects of neuregulin-1β on growth-associated protein 43 expression in dorsal root ganglion neurons with excitotoxicity induced by glutamate in vitro. Neurosci Res 76:22-30.
Liu Z, Cai H, Zhang P, Li H, Liu H, Li Z (2012) Activation of ERK1/2 and PI3K/Akt by IGF-1 on GAP-43 expression in DRG neurons with excitotoxicity induced by glutamate in vitro. Cell Mol Neurobiol 32:191-200.
Liu Z, Li H, Zhang W, Li Y, Liu H, Li Z (2011) Neuregulin-1β prevents Ca(2+) overloading and apoptosis through PI3K/Akt activation in cultured dorsal root ganglion neurons with excitotoxicity induced by glutamate. Cell Mol Neurobiol 31:1195-1201.
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Saleh A, Roy Chowdhury SK, Smith DR, Balakrishnan S, Tessler L, Martens C, Morrow D, Schartner E, Frizzi KE, Calcutt NA, Fernyhough P(2013) Ciliary neurotrophic factor activates NF-κB to enhance mitochondrial bioenergetics and prevent neuropathy in sensory neurons of streptozotocin-induced diabetic rodents. Neuropharmacology 65:65-73.
Sango K, Yanagisawa H, Takaku S (2007) Expression and histochemical localization of ciliary neurotrophic factor in cultured adult rat dorsal root ganglion neurons. Histochem Cell Biol 128:35-43.
Selvaraj BT, Sendtner M (2013) CNTF, STAT3 and new therapies for axonal degeneration: what are they and what can they do? Expert Rev Neurother 13:239-241.
Siegel SG, Patton B, English AW (2000) Ciliary neurotrophic factor is required for motoneuron sprouting. Exp Neurol 166:205-212.
Takanashi J, Mizuguchi M, Terai M, Barkovich AJ (2015) Disrupted glutamate-glutamine cycle in acute encephalopathy with biphasic seizures and late reduced diffusion. Neuroradiology 57:1163-1168.
Tsai SY, Yang LY, Wu CH, Chang SF, Hsu CY, Wei CP, Leu SJ, Liaw J,Lee YH, Tsai MD (2007) Injury-induced Janus kinase/protein kinase C-dependent phosphorylation of growth-associated protein 43 and signal transducer and activator of transcription 3 for neurite growth in dorsal root ganglion. J Neurosci Res 85:321-331.
Vieira AS, Rezende AC, Grigoletto J, Rogério F, Velloso LA, Skaper SD,Negro A, Langone F (2009) Ciliary neurotrophic factor infused intracerebroventricularly shows reduced catabolic effects when linked to the TAT protein transduction domain. J Neurochem 110:1557-1566.
Yin DP, Chen QY, Liu L (2016) Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation). Neural Regen Res 11:1006-1012.
Zhou HL, Zhang LS, Kang Y, Zhang W, Wang TH (2008) Effects of electro-acupuncture on CNTF expression in spared dorsal root ganglion and the associated spinal lamina II and nucleus dorsalis following adjacent dorsal root ganglionectomies in cats. Neuropeptides 42:95-106.
Zhou WD, Wang LL, Zhou LB, Bin W, Bao TP, Zhang Y, Shu J, Yang WX,Hui LL, Jin R, Zhuang LL, Zhou GP (2017) All-trans retinoic acid upregulates the expression of ciliary neurotrophic factor in retinal pigment epithelial cells. Cell Biochem Funct doi: 10.1002/cbf.3264.
Graphical Abstract
Ciliary neurotrophic factor (CNTF) alleviates glutamate-induced excitotoxicity in cultured dorsal root ganglion
*Correspondence to:
Yi Xing, M.D., Ph.D.,xingyi2016@yahoo.com.
orcid:
0000-0001-7594-8398
(Yi Xing)
10.4103/1673-5374.217352
Accepted: 2017-08-20
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
 中國(guó)神經(jīng)再生研究(英文版)2017年10期
中國(guó)神經(jīng)再生研究(英文版)2017年10期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Diffusion tensor tractography studies on mechanisms of recovery of injured fornix
- Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration
- Effect of glial cells on remyelination after spinal cord injury
- miR-30c promotes Schwann cell remyelination following peripheral nerve injury
- End-to-side neurorrhaphy repairs peripheral nerve injury: sensory nerve induces motor nerve regeneration
- Central projections and connections of lumbar primary afferent fibers in adult rats: effectively revealed using Texas red-dextran amine tracing
