Preparation and characterization of solidifed oleanolic acid–phospholipid complex aiming to improve the dissolution of oleanolic acid
School of Pharmacy,Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
Preparation and characterization of solidifed oleanolic acid–phospholipid complex aiming to improve the dissolution of oleanolic acid
Xiaoxu Yang,Qikun Jiang,Ping Du,Juanhang Zhao,Tianhong Zhang*
School of Pharmacy,Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
A R T I C L EI N F O
Article history:
Received 17 May 2015
Received in revised form 30 June 2015
Accepted 12 July 2015
Available online 28 August 2015
Oleanolic acid
The purpose of this study was to prepare the oleanolic acid–phospholipid complex(OAPC)and then solidify it employing fumed silica by simple solvent evaporation technique to improve dissolution rate of oleanolic acid and oleanolic acid–phospholipid complex.The process of OA-PC was optimized and the type and proportion of fumed silica were studied by dissolution text.The structures of the phospholipid complex and solidifed powder were also characterized by differential scanning calorimetry,X-ray diffraction,and scanning electron microscope.In the dissolution tests,OA from solidifed powder was further released compared with that from pure OA and OA-PC in different kinds of dissolution media.These results suggest that the method of preparing solidifed powder of oleanolic acid–phospholipid complex is suitable for enhancing the dissolution rate of OA and OA-PC.
?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical
University.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Oleanolic acid,3β-3-hydroxyolean-12-en-28-oic acid(OA,Fig.1), is a triterpenoid compound that exists widely in many Asian herbs,such as Fructus ligustri lucidi,Fructus orsythiae,and Akebia trifoliate.OA has multiple pharmaceutical functions such as anti-infammatory,enhancement of human body defense systems and hypoglycemic[1].In clinical,OA is available over the counter in tablet form and has been used for several decades as an adjunct therapy for hepatitis[2].However,OA is a class IV drug according to the Biopharmaceutics Classifcation System [3],and has an absolute oral bioavailability of only 0.7%because of its low permeability(Papp=1.1?1.3×10?6cm/s in the apicalto-basolateral direction at 10 and 20 μM)[4]and low aqueous solubility(<1 μg/ml)[5].This has restricted the use of varieties of pharmacological activities of OA for treating different disorders.
Several techniques had been attempted to improve the dissolution of OA,for example,preparation of different crystal forms of OA by recrystallization[5],preparing solid dispersion[6],nanoemulsions[7]and nanosuspensions[8].However, solid dispersion and nanosuspensions focused on the dissolution improvement of OA and paid no attention to the poor permeability of oleanolic acid,another potential root-cause of low bioavailability.In the case of nanoemulsions,the complextechnology and large amount of excipients added were a large challenge.Thus,the proper formulation of oleanolic acid should improve not only solubility and dissolution but also membrane permeability of OA.Recently,the drug–phospholipid complex technique is demonstrated as a promising method to improve membrane permeability and water solubility,thereby enhancing bioavailability for BCSIV drugs[9–12].
Phospholipids are an important component of the cell membrane which can penetrate the cell membrane easily.Besides, it also treats hepatic disorder.Today,phospholipids are widely used as active ingredients and pharmaceutical excipients.The drug–phospholipid complex can improve membrane penetration potential and absorption of drugs,which enhance the bioavailability of BCSIV drugs[13,14].
However,the drug–phospholipid complex also has large stickiness,which would pose a challenge for drug dissolution,further processing into other formulations and industrial production.Thus,appropriate carriers might be employed to solidify the drug–phospholipid complex in order to lower stickiness of OA-PC.Fumed silica has a high specifc surface area and porosity,strong adsorption ability and good wetting properties,and it is usually used as a carrier to improve dissolution of poorly soluble drugs[15,16].Consequently,we studied the effects of different types of fumed silica on dissolution of OA.
In the present studies,novel solidifed powder of the oleanolic acid–phospholipid complex was frst prepared using solvent evaporation method based on the OA-PC.The prepared OA-PC and solidifed powder were evaluated by thermal behavior(DSC),X-ray diffraction(XRD)and scanning electron microscope(SEM).Moreover,dissolution profles of the novel solidifed powders were compared with pure OA and OA-PC.
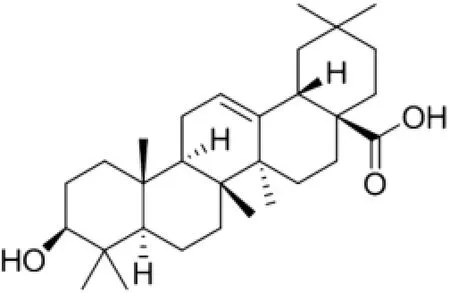
Fig.1–The chemical structures of OA.
2.Materials and methods
2.1.Materials
OA(purity of 98.0%)was purchased from Shikun Sichuan Pharmaceutical Co.Ltd.Lipoid E200(trade name of phospholipid, purity 80%)was purchased from Shanghai Advanced Vehicle Technology Co.Ltd.Fumed Silica(EY-QX1)was purchased from Shanhe Anhui Pharmaceutical Co.Ltd.Fumed Silica(CAB-OSIL M-5,USA;AEROSIL 380,USA)was supplied by Beijing Fengli Jingqiu Commerce and Trade Co.Ltd.Methanol(chromatographic grade)was purchased from Kangkede Tianjin Co.Ltd (China).Phosphate(chromatographic grade)was supplied by KemioTianjin Co.Ltd(China).The other chemical reagents were of analytical grade or better.
2.2.Preparation of the OA-PC and solidifed powders by solvent-evaporation method
2.2.1.Screening the best molar proportion of phospholipidsand drug
The drug and phospholipid at the molar ratios of 1:0.5,1:1,1:2 and 1:3 were accurately taken into a 100 ml round bottom fask and 20 ml anhydrous ethanol was added then refuxed at 60°C for 2 h.The anhydrous ethanol was removed by rotary evaporator at 40°C.The dried residues were gathered and dried in desiccators overnight,and then sieved with an 80 mesh.To obtain the optimal molar ratio of OA to phospholipid,the solubility of the drugs in phospholipid complexes was determined using HPLC method.According to the results of the solubility assay,the optimum molar ratio of OA and phospholipid was determined.
2.2.2.Screening the best reaction temperature of phospholipids and drug
The drug and phospholipid at the molar ratio of 1:1 were taken into a 100 ml round bottom fask and dissolved in anhydrous ethanol.The mixture was refuxed under 30,40,60 and 80°C for 2 h,respectively.Then the anhydrous ethanol was removed by rotary evaporator at 40°C.The dried residues were placed in desiccators overnight,then sieved with an 80 mesh and stored at room temperature.To choose the best reaction temperature,the water solubility of OA in prepared OA-PC complex was determined using HPLC method.Based on the results of the solubility study,we determine the best reaction temperature of phospholipids and drug.
2.2.3.Screening the best reaction time of phospholipids anddrug
The drug and phospholipid at the molar ratio of 1:1 were taken into a 100 ml round bottom fask and dissolved in anhydrous ethanol.The mixture was refuxed under 60°C for 0.5,1,1.5, 2,3 h respectively.Then the anhydrous ethanol was removed by rotary evaporator at 40°C.The dried residues were placed in desiccators overnight,then sieved with an 80 mesh and stored at room temperature.Then the solubility of prepared OA-PC complex was studied and the best reaction time of phospholipids and drug was determined.
2.2.4.Screening the kinds of fumed silica
The drug and phospholipid at the molar ratio of 1:1(account to 1:2,w/w)were taken into a 100 ml round bottom fask and dissolved in anhydrous ethanol.The mixture was refuxed under 60°C for 2 h.Then same amount of different kinds of fumed silica(CAB-O-SIL M-5,AEROSIL 380 and EY-QX1)was added into the above solution and constantly stirred for a period of time. Then the anhydrous ethanol was removed by rotary evaporator at 40°C.The dried residues were placed in desiccators overnight,then sieved with an 80 mesh and stored at room temperature.According to the results of the dissolution test, the favorable kinds of fumed silica were determined.
2.2.5.Screening the optimal proportion of OA and fumed silica
The drug and phospholipid at the molar ratio of 1:1(account to 1:2,w/w)were taken into a 100 ml round bottom fask anddissolved in anhydrous ethanol.The mixture was refuxed under 60°C for 2 h.Then fumed silica in different mass ratios(OA: fumed silica=1:3;1:5;1:7;1:9)were added into the above solution respectively,and constantly stirred for a period of time. Then the anhydrous ethanol was removed by rotary evaporator at 40°C.The dried residues were placed in desiccators overnight,then sieved with an 80 mesh and stored at room temperature.Based on the dissolution results,we were able to determine the optimal proportion of OA and fumed silica.
2.3.Differential scanning calorimetric analysis(DSC)
DSC curves were carried out with a Mettler DSC 30S(Mettler Toledo,Switzerland).The samples including pure OA,phospholipid,OA-PC,fumed silica,physical mixture of OA,PC,and fumed silica and solidifed powder were sealed into aluminum pans separately and heated from 40 to 350°C,at a heating rate of 10°C/min.
2.4.X-ray diffraction(XRD)
The crystalline state of pure OA,phospholipid,OA-PC,fumed silica,physical mixture and solidifed powder were measured at room temperature on D/max-r A(Rigaku Denki,Japan) equipped with a Cu Ka radiation source at 45 kV and 30 mA. The text was carried out from 2°to 50°in the range of 2θ.
2.5.Scanning electron microscopy(SEM)
SEM(SU 8000,Hitachi,Japan)was employed to observe the morphology of pure OA,phospholipid,OA-PC,fumed silica,physical mixture and solidifed powder In brief,samples were fxed on an aluminum stub with conductive double-sided-adhesive tape and then coated with gold.The surface morphology of the samples was examined.
2.6.Solubility studies
Solubility determination of OA in OA-PC complex was carried out by adding excess of the samples to 5.0 ml of water in a sealed glass container.Each sample was performed in triplicate.Then the liquids were shaken to balance in the swing bed at 37°C.The liquid was centrifuged at 15,000 rpm for 10 min and the supernatant was fltered through a 0.45 μm membrane.A 20 μL aliquot of the fltrate was injected into HPLC system directly.
2.7.Dissolution profle
The dissolution tests of OA from OA-PC and solidifed powder were evaluated using the Chinese pharmacopoeia method II (paddle method)with a ZRS-8G dissolution apparatus (Tiandatianfa,Tianjin,China).Dissolution was conducted in 900 ml different concentrations of sodium dodecyl sulfate(SDS) aqueous solution to satisfy the sink conditions.We chose 0.3% sodium dodecyl sulfate(SDS)aqueous solution as the dissolution medium to determine the optimal solidifed carrier and its proportion in powders.The paddle speed was set to 100 rpm and the water bath temperature was maintained at 37±0.5°C. OA,OA-PC and solidifed powders which were equivalent to 20 mg OA were added to the dissolution medium.The dissolution medium of 5 ml was withdrawn after 5,10,20,30,45, 60,90 and 120 min,and replaced with the same medium volume.The withdrawn samples were fltrated(0.45 μm)and analyzed by HPLC at 210 nm.
2.8.HPLC analysis
All the involved samples were analyzed by HPLC(Shimadzu, Japan)on a C18 column(Thermo C18,4.6×150 mm,5 μm,USA) and the column was kept at room temperature.The mobile phase was methanol–water–phosphate(90:10:0.01 v/v/v)and the fow rate was 1.0 ml/min.The wavelength of the UV detector was set at 210 nm.
3.Results and discussion
3.1.The best molar ratio of OA to phospholipid
According to the article,the process of phospholipid complex is usually evaluated by the combination ratio of drug and phospholipids[17].However,in this study,OA has a good lipophilicity and it is diffcult to fnd a suitable solvent to calculate the combination ratio of OA and phospholipid.Thus,the variation of solubility of OA-PC was chosen to evaluate the process of phospholipid complex.In Fig.2 we can see that,under the same reaction temperature and time,the solubility of OPC is improved with the increased proportion of phospholipids in complex.Considering that the variation of solubility among the molar ratios of 1:1,1:2 and 1:3 was not very distinguished, the optimal molar ratio of drug to phospholipid was selected at 1:1.
3.2.The best reaction temperature of phospholipids and drug
Based on the results presented in Fig.3,the solubility of OAPC was increased with the variation of reaction temperature from 30 to 60°C,which showed that the formation of phospholipid complex could be positively correlated with reactiontemperature.When the temperature was increased to 80°C, solubility of OA-PC reduced.This might be explained by the oxidation of phospholipid due to high temperature.Thus,60°C was chosen as the optimal reaction temperature in this study.
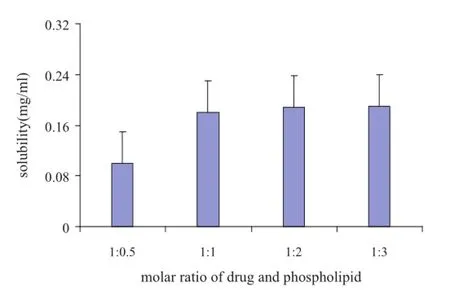
Fig.2–The solubility of OA in OA-PC at different molar ratios of OA to phospholipid.
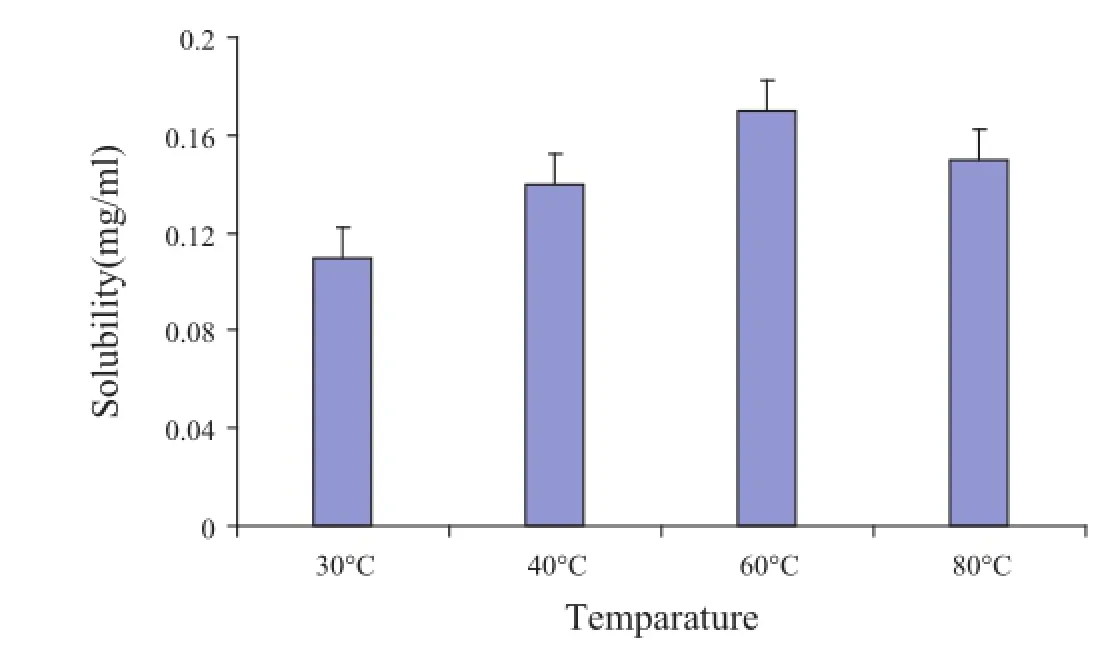
Fig.3–The solubility of OA in OA-PC at different reaction temperatures.
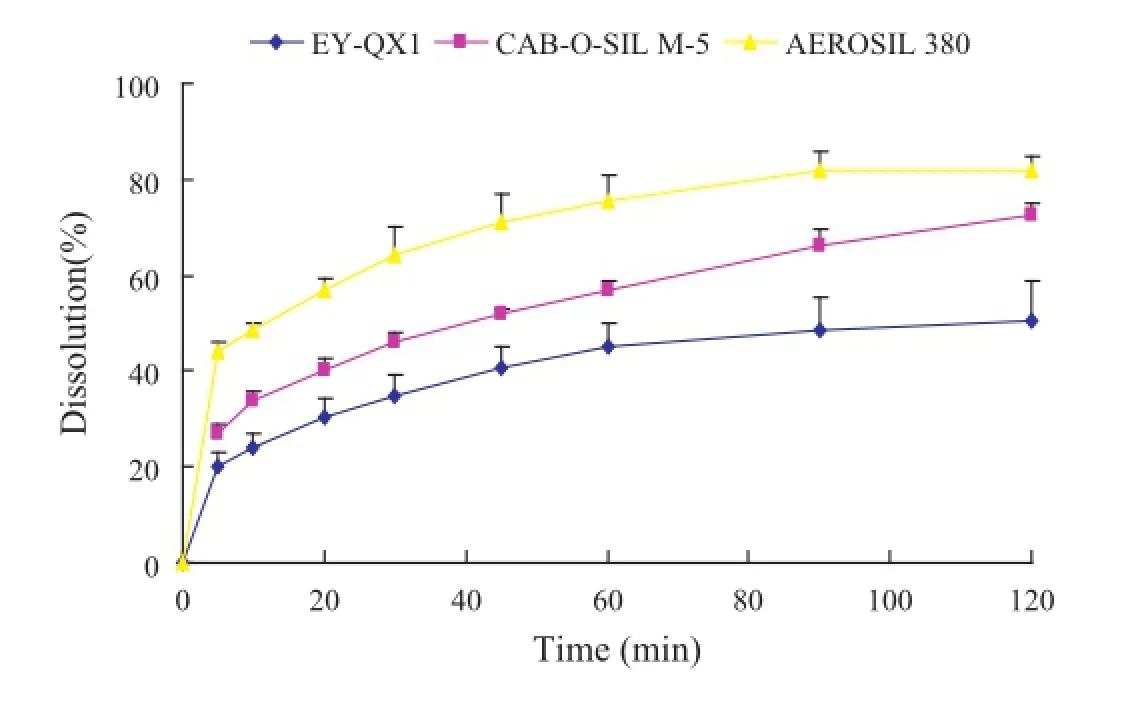
Fig.5–The dissolution profles of the OA-PC-solidifed powders prepared by different types of fumed silica in 0.3% SDS aqueous solution.
3.3.The best reaction time of phospholipids and drug
Fig.4 shows that the solubility of OA-PC was enhanced with the increase in reaction time from 0.5 to 2 h.However,after 2 h reaction was done,the solubility of OPC was not increased with the variation of time.As a result,the reaction time of 2 h is most appropriate in the process of phospholipid complex.
3.4.The best kinds of fumed silica
To improve the poor fowability and dissolution of OA-PC,we further prepared the solidifed powders of OA-PC employing different kinds of fumed silica including CAB-O-SIL M-5(specifc surface area 200±25 m2/g,and particles 12 nm),AEROSIL 380(specifc surface area 380±30 m2/g,and particles 7 nm)and EY-QX1(particles 15 nm).As shown in Fig.5,the properties of the solidifed carriers played an important role in the dissolution profle of the drug.These kinds of solidifed powdersexhibited higher dissolution rate compared with the pure OA and OA-PC.Furthermore,due to the higher specifc surface area and lower particle size,the solidifed powder using AEROSIL 380 as solidifed carrier displayed higher dissolution compared with the other kinds of solidifed powders.
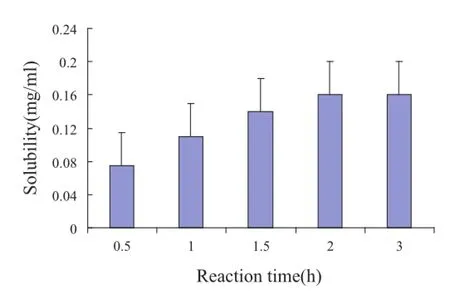
Fig.4–The solubility of OA in OA-PC at different reaction times.
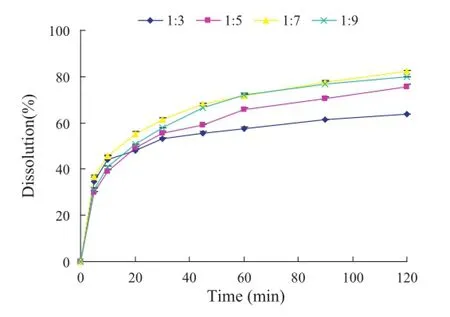
Fig.6–The dissolution profles of the solidifed powders with different mass ratios of OA to fumed silica in 0.3% SDS aqueous solution.
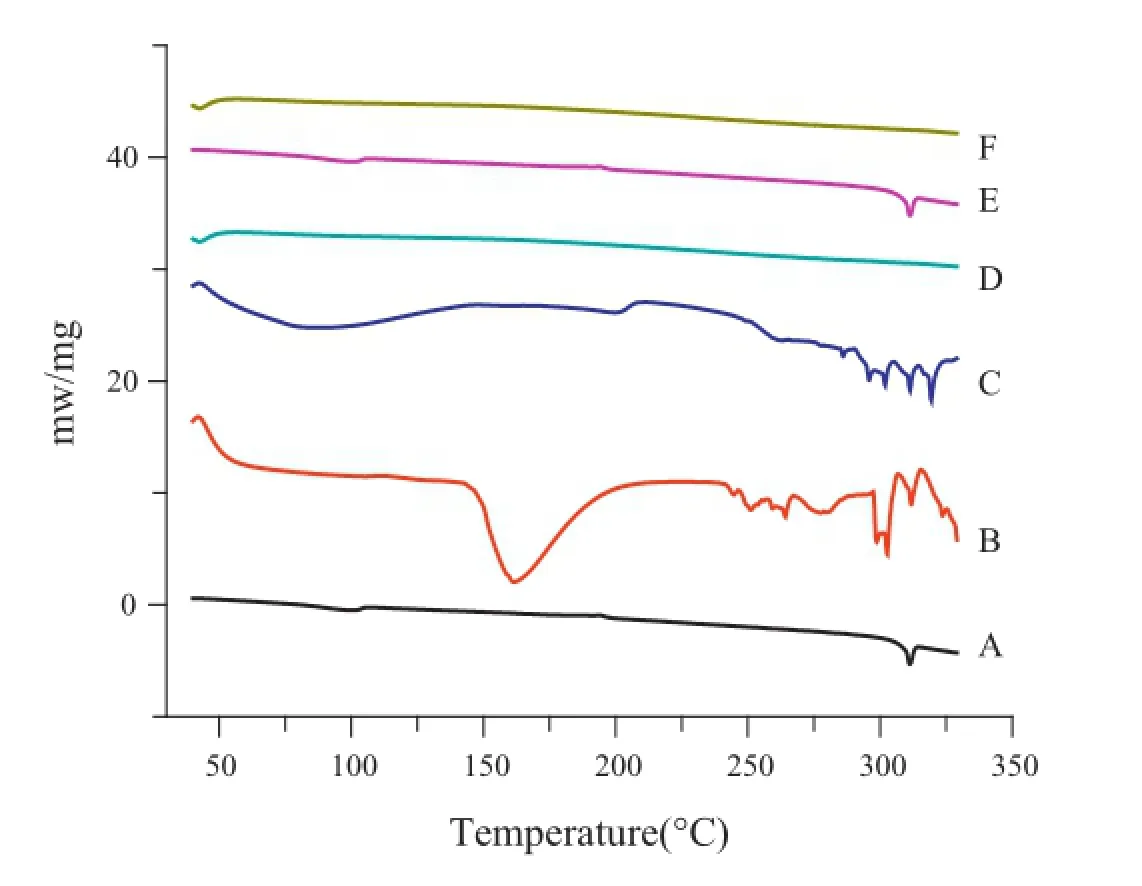
Fig.7–DSC spectra of(A)OA,(B)phospholipid,(C)OA-PC, (D)fumed silica,(E)physical mixture,and(F)solidifed powder.
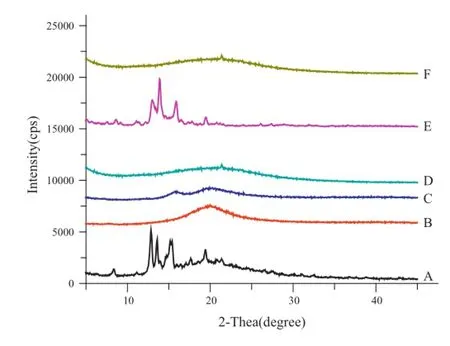
Fig.8–X-ray diffractograms of(A)OA,(B)phospholipid, (C)OA-PC,(D)fumed silica,(E)physical mixture,and (F)solidifed powder.
3.5.The best proportion of OA and fumed silica
The best proportion of fumed silica in solidifed powders was studied by dissolution test and the results were shown in Fig.6. From the fgure,we can see that the solidifed powders of OAPC with mass ratio of OA to fumed silica of 1:7 exhibited the highest dissolution rate.When fumed silica increased,dissolution rates were not improved.Consequently,the mass ratio of OA to fumed silica of 1:7 was the best.
3.6.DSC
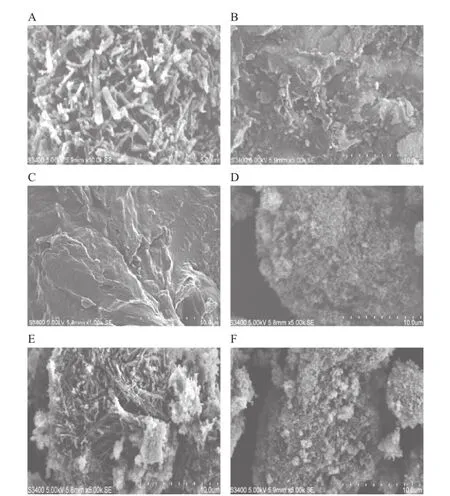
Fig.9–SEM images of(A)OA,(B)phospholipid,(C)OA-PC,(D)fumed silica,(E)physical mixture,and(F)solidifed powder.
DSC study is an important method to reveal compatibility between drugs and excipients and provides large informationabout possible interactions[18].Fig.7 shows the DSC thermograms of original OA(A),phospholipid(B),OA-PC(C),fumed silica(D),physical mixture of OA,phospholipid and fumed silica (E)and solidifed powder(F).An endothermic peak was found at 312°C for the crude OA and physical mixture,indicative of its anhydrous and crystalline state.On the contrary,both in the OA-PC and solidifed powders,the endothermic peak of OA substance disappeared,indicating the amorphous form of OA in OA-PC and solidifed powders.
3.7.XRD
Fig.8 displays the X-ray diffraction patterns of original OA(A), phospholipid(B),OA-PC(C),fumed silica(D),physical mixture of OA,phospholipid and fumed silica(E)and solidifed powder (F).In the X-ray diffractogram,OA material showed intense diffraction peaks suggesting that the drug is present as a crystalline material.In physical mixture,the diffraction peaks were reduced but can still be observed easily,indicative of the presence of a crystal form of OA.On the other hand,there were no diffraction peaks of OA observed in OA-PC and solidifed powders,which showed that OA presents an amorphous state in the OA-PC and solidifed powders.These data support the DSC studies which indicated the reduced crystallinity of drugs in OA-PC and solidifed powder.
3.8.SEM
As shown in Fig.9A,the morphology of the pure OA showed that it was in needle-like crystal.It was regular in shape and size.Furthermore,needle-like crystals can be observed in the physical mixture shown in Fig.9E.From Fig.9C and F,we can conclude that OA showed an amorphous state in the OA-PC and solidifed powders,indicating the formation of OA-PC and solidifed powders,which was in agreement with the results of the XRD and DSC.
3.9.Dissolution study
The dissolution profles of original OA,OA-PC and solidifed powders in different dissolution media are plotted in Fig.10. Because of the viscous state of the phospholipid complex,the dissolution of the OA-PC complex was less than crude OA. However,the dissolution rate of the prepared solidifed powder using fumed silica as carriers was faster than that of pure OA and OA-PC.These could be explained as follows:1)OA was in an amorphous state in solidifed powders,which is a more soluble form;2)fumed silica with high specifc surface area had a good wetting and adsorption ability and was usually served as a carrier to improve the dispersion of poorly soluble drugs.
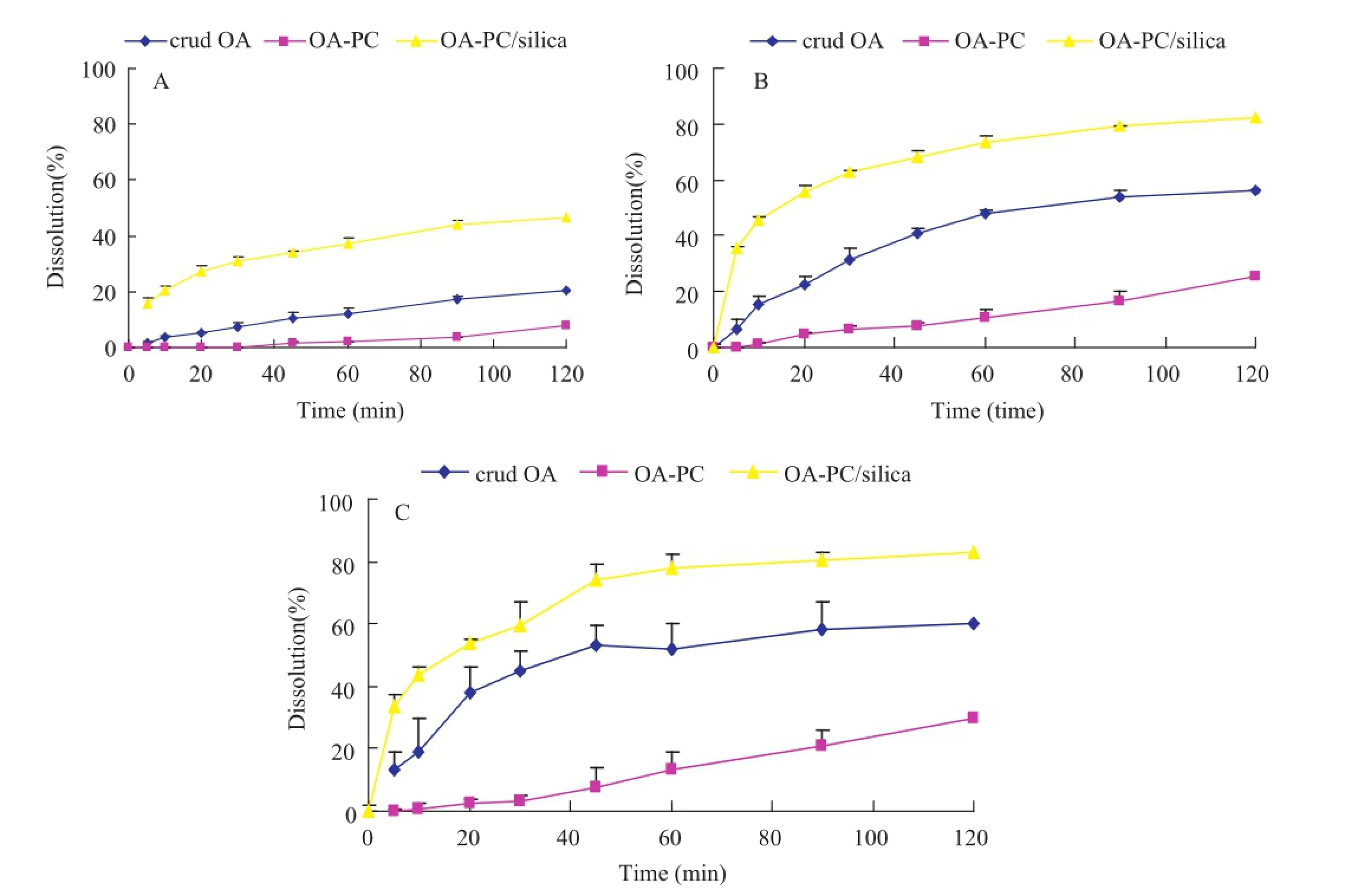
Fig.10–Dissolution profles of OA from crude OA,phospholipid complex(OA-PC)and solidifed powders(OA-PC/silica)in (A)0.1%SDS aqueous solution;(B)0.3%SDS aqueous solution;(C)0.5%SDS aqueous solution.
4.Conclusions
The phospholipid complex technology can improve the bioavailability and oral absorption of BCSIV drugs.However, the stickiness of the phospholipid complex might limit dissolution of drugs and made it diffcult to prepare other formulations.In this paper,solidifed powder of OA-PC was prepared by a simple and feasible solvent method and it has been proved to be able to improve the dissolution of OA and OAPC.Moreover,based on the solidifed powder,we can further prepare the dosage form that is easy to take and produce,which could further develop the clinical application of OA.As a result, solidifed powder of OA-PC would be a prospective and practical drug formulation.
Acknowledgments
The authors are very grateful to the Analytical Department of Shenyang Pharmaceutical University.
R E F E R E N C E S
[1]Zhou Z,Yuan D.Research development of pharmacologic action of oleanolic acid.Chin J Hosp Pharm 2008;28:2031–2033.
[2]Hu H.Research development of pharmacologic action and clinical application of oleanolic acid.J Haix Pharm 2012;24:92–94.
[3]Amidon GL,Lennern?s H,Shah VP,et al.A theoretical basis for a biopharmaceutics drug classifcation:the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 1995;12:413–420.
[4]Jeong DW,Kim YH,Kim HH,et al.Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats.Biopharm Drug Dispos 2007;28:51–57.
[5]Tong HH,Wu HB,Zheng Y,et al.Physical characterization of oleanolic acid nonsolvate and solvates prepared by solvent recrystallization.Int J Pharm 2008;355:195–202.
[6]Neu VT,Zhao HR.Preparation of oleanolic acid solid dispersion systems and their dissolution in vitro.J Chin Pharm Univ 2003;34:236–239.
[7]Alvarado HL,Abrego G,Souto EB,et al.Nanoemulsions for dermal controlled release of oleanolic and ursolic acids:in vitro,ex vivo and in vivo characterization.Colloids Surf B Biointerfaces 2015;130:40–47.
[8]Li W,Das S,Ng K,et al.Formulation,biological and pharmacokinetic studies of sucrose ester-stabilized nanosuspensions of oleanolic acid.Pharm Res 2011;28:2020–2033.
[9]Li J,Liu P,Liu J-P,et al.Bioavailability and foam cells permeability enhancement of salvianolic acid B pellets based on drug–phospholipids complex technique.Eur J Pharm Biopharm 2013;83:76–86.
[10]Wu H,Long X,Yuan F,et al.Combined use of phospholipid complexes and self-emulsifying microemulsions for improving the oral absorption of a BCS class IV compound, baicalin.Acta Pharm Sin B 2014;4:217–226.
[11]Khan J,Alexander A,Ajazuddin,et al.Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profle of plant actives.J Control Release 2013;168:50–60.
[12]Zhang Z,Chen Y,Deng J,et al.Solid dispersion of berberine–phospholipids complex/TPGS1000/SiO2:preparation, characterization and in vivo studies.Int J Pharm 2014;465:306–316.
[13]Ma H,Chen H,Sun L,et al.Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia 2014;93:54–61.
[14]Guo B,Liu H,Li Y,et al.Application of phospholipid complex technique to improve the dissolution and pharmacokinetic of probucol by solvent-evaporation and co-grinding methods.Int J Pharm 2014;474:50–56.
[15]Sanganwar GP,Gupta RB.Dissolution-rate enhancement of fenofbrate by adsorption onto silica using supercritical carbon dioxide.Int J Pharm 2008;360:213–218.
[16]Smirnova I,Suttiruengwong S,Seiler M,et al.Dissolution rate enhancement by adsorption of poorly soluble drugs on hydrophilic silica aerogels.Pharm Dev Technol 2004;9:443–452.
[17]Ding D,Zhang Z,Jiang Y,et al.Advance in studies on phospholipid compound of traditional Chinese medicines. China J Chin Mater Med 2013;38:2046–2050.
[18]Maiti K,Mukherjee K,Gantait A,et al.Curcumin–phospholipid complex:preparation therapeutic evaluation and pharmacokinetic study in rats.Int J Pharm 2007;330:155–163.
*< class="emphasis_italic">Corresponding author.
.Department of Pharmaceutical Analysis,School of Pharmacy,Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Tel.:+86 24 23984159;fax:+86 24 23986321.
E-mail address:zhangth_student@aliyun.com(T.Zhang).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.07.002 1818-0876/?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Phospholipid complex
Fumed silica
Dissolution
 Asian Journal of Pharmacentical Sciences2016年2期
Asian Journal of Pharmacentical Sciences2016年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- Ameliorated effects of Lactobacillus delbrueckii subsp. lactis DSM 20076 and Pediococcus acidilactici NNRL B-5627 on Fumonisin B1-induced Hepatotoxicity and Nephrotoxicity in rats
- Validation and application of Vierordt’s spectrophotometric method for simultaneous estimation of tamoxifen/coenzyme Q10 in their binary mixture and pharmaceutical dosage forms
- Introduction of antineoplastic drug NSC631570 in an inpatient and outpatient setting: Comparative evaluation of biological effects
- Control of autoimmune arthritis by herbal extracts and their bioactive components
- The 1st Euro-Mediterranean Workshop:Natural Products in Health and Diseases:Cairo,Egypt, March 2,2015
- Effect of process parameters on the recrystallization and size control of puerarin using the supercritical fuid antisolvent process
