Relative Content of Phenoxyethanol and p-Hydroxyacetophenone in Water Phase of Oil-water Systems and Teir Relevant Control Parameters
Xiaoling Qiao*, Ping Cao*, Yuanhua Cong, Chunbo Feng, Zhitao Chen,Xuepu Li, Lingling Zhang, Qin Zhu, Zheng Wang, Xinran Li
Shanghai Jahwa United Co., Ltd., China
Introduction
Preservatives have attracted enough research interests in cosmetics, food, pharmaceuticals fields during the past decades.[1-4]Recently, related topics in cosmetic research area become more and more popular. This trend is not only related to preservative protection ability to against microbial spoilage, but also to lots of safety concerns raised up by consumers.[1,2,5,6]Statistical experimental data from hospital show that skin care, hair care and bodycleansing products were the top three products which might have caused allergic contact dermatitis among eleven different categories of cosmetic products.[7,8]Further analysis and studies reveal that fragrance and preservatives play major negative roles as allergens.[7-9]Official organizations also have paid enough attentions to this problem. European, China and US et. al. have established many related standards or laws to set limitation or ban some preservatives, such as iodopropynyl butylcarbamate(IPBC), methylisothiazolinone (MIT), kathon CG,parabens and so on.[5,10]Accordingly, some sensitive cosmetic brands capture this consumer insight and further claim out “no preservative” or “preservative-free”to reassure existing and attract new consumers. Actually,back to the nature of research, the key point to solve these related problems is finding the balance between preservative effectiveness and product safety. Aimed for this purpose, three new research fashions are mainly focused from chemist viewpoint: (I) synthesize or look for new preservative molecules; (II) develop preservativefree formula; (III) discover the minimum dosage which can provide preservative function. Actually, there are also some other solutions through packaging approaches,such as vacuum pump, capsule package, et. al., however, it won’t be the discussed here.
Synthesis or looking for new preservative molecules are the heated research topics among the three directions.These new preservatives could be classified to polyols,aromatic compounds, aliphatic compounds, organic acids and plant-derived essential oils and extracts.[4]Among these new ingredients, polyols and plant derived essentials attracts enough attentions. Both of them are the favorite choices to formulators as their good images in consumers’ mind. Furthermore, most of these ingredients are not classified to be preservatives, which could be utilized by some cosmetic brand to call out“preservative-free” related claims. Polyols own many good candidates to provide antimicrobial spoilage. Due to their amphiphilic chemical structure, they interfere with cellular structures of microorganisms and disintegrate cell membranes. Glyceryl caprylate has been reported to be used as an antimicrobial substance alone or in combination with other antimicrobial compounds for cosmetics preservation. Caprylyl glycol, 1,2-pentanediol and hexylene glycol have also been used in formulations,gradually.[4,5]A number of plant-derived essential oils and extracts also have exhibited antimicrobial properties and have further been used for the effective preservation of cosmetic formulations.[5,11,12]Except for these new“preservatives”, some other antioxidant and chelating agents are also found to have very good preservation or preservation enhancer effects,[5]such as EDTA disodium,ethylhexylglycerin and p-Hydroxyacetophenone (p-HAP).
Regarding to realize designing no preservative containing products, the core strategy is to reduce water activity of the formulation.[12]Generally, traditional approaches are to make pure oil, high percentage of salt,alcohol or polyols containing formulation base. Of course,these formulation prototypes have many limitations to be applied in final products to satisfy consumer needs, especially in skincare products. Preservative-free aqueous formulations can also be made microbiologically stable by sterile production and appropriate packaging.However, this approach may not work for the most aqueous cosmetics packaged in multiple-use containers.[5]Recently, a creative “frozen-dry” technology has emerged in cosmetic field which has inspired from pharmaceutical technology. This creative idea is to freeze formulation by liquid nitrogen and remove water by sublimation in vacuum to produce dry form products to realize no preservative.

No matter preservatives are traditional or new creative versions, content of preservatives should be close to the minimum effective dose in formulation if possible. This is a classical approach, but effective and necessary to improve product safety. Formulation pH, water activity,and ethanol have been reported as the three well-known parameters which influence preservation.[13,14]In surfactant system, surfactant micelle in shampoo, shower gel could also weaken preservation ability. While in emulsion base,reducing emulsion droplets size is also negative to against microbial spoilage.[4,15]Given that all micro-organism survive only in a water environment, the concentration of preservative in the water phase is directly related to the micro-organism control in the cosmetic system. The amount of a preservative dissolved in the oil phase could be treated as a “l(fā)oss” for the preservative. Therefore, it is desirable to know the partitioning of a preservative between oil and water phases in practical application.
Phenoxyethanol (PE) and p-HAP system is newly proposed preservative pair, whose chemical structures are shown in Figure 1, respectively. It becomes a good candidate to replace some limited preservatives such as parabens, Kathon CG, IPBC, MIT et. al. It has been gradually emerged in many new launching products. However, this system still has limited additive percentage due to its preservation ability will naturally cause irritation. Thus increasing the effective content of preservatives in water phase and decreasing waste preservatives in oil phase are the key approaches to reduce potential hazard. In this communication, the new preservative system will be selected as research objective. Following concentrations of PE and p-HAP in water phase will be determined directly to reflect the effective content of preservatives. Relative content (C)of PE or p-HAP (CPEand Cp-HAP) is new defined to stand for the percentage of PE or p-HAP is located in water phase. Both CPEand Cp-HAPin eight different oil-water phases are further checked carefully. Besides, polyols and processing parameter effects on CPEand Cp-HAPare also investigated. Through these approaches, we hope results and discussions in this work could provide any reference for formulation and processing scientists to find the balance between preservation and safety in reality.
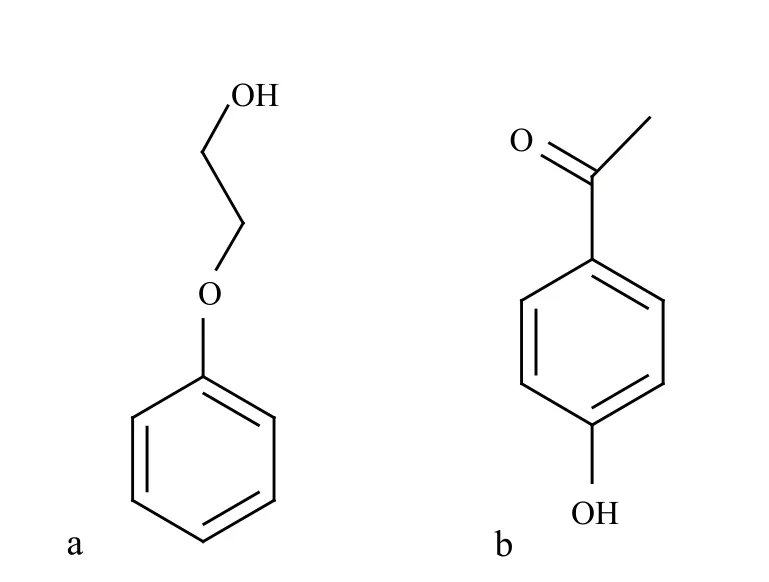
Figure 1. Chemical structure of PE (a) and p-HAP (b)
Materials and method
Materials and instruments
Phenoxyethanol (PE), p-Hydroxyacetophenone(p-HAP), 1,3-butylene glycol (BG), squalane, petrolatum,caprylic/capric triglyceride (GTCC), silicone oil (200cst),olive oil, hydrogenated polyisobutene, cetostearyl alcohol and isononyl isononanoate were all cosmetic grade and used directly without any further treatment. Polytron PT 3100D was purchased from Kinematica to apply homogenization.
Experimental method and procedure
The formulation will be listed in Table 1. Two experimental procedures are designed to be more closely to reality manufacture processing. Both of them will be described as follows.
Route A is drawn in Figure 2(a). Firstly, Phase A, B, C and D will be heated up to 80oC. Until both of them are melted separately, four phases will be added into main homogenization with a speed of 3,000 rpm at 80oC for a certain time. After the homogenization is finished, the unstable system will be kept at room temperature for 24 hours.
Route B is presented in Figure 2(b). Firstly, Phase A, B, C and D are heated up to the temperature of 80oC to ensure all the components are in molten state. Secondly, Phase A waspoured into Phase B under homogenization with a speed of 3,000 rpm at 80oC for 5 mins. Thirdly, the mixture of Phase A and B was cooled down to 50oC. Fourthly, Phase C and D were transferred into main beaker. Finally, homogenization was applied and kept for 30 min. Then, the mixture was cool down to room temperature and kept for 24 h.
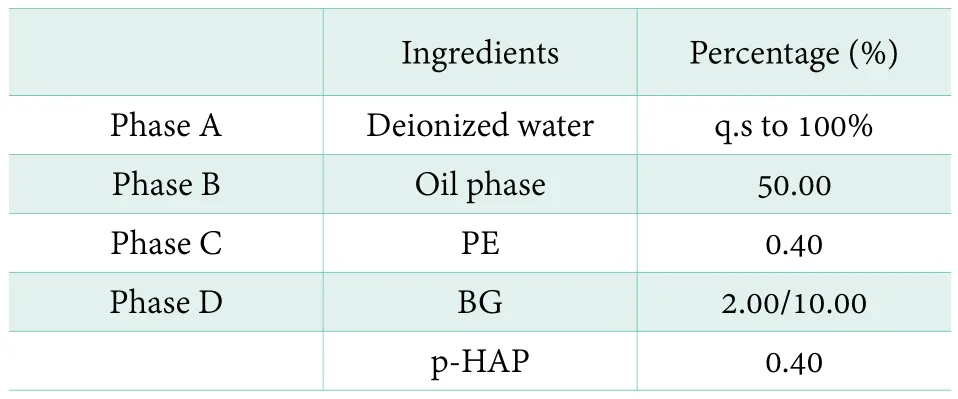
Table 1. The formulation of the studied system
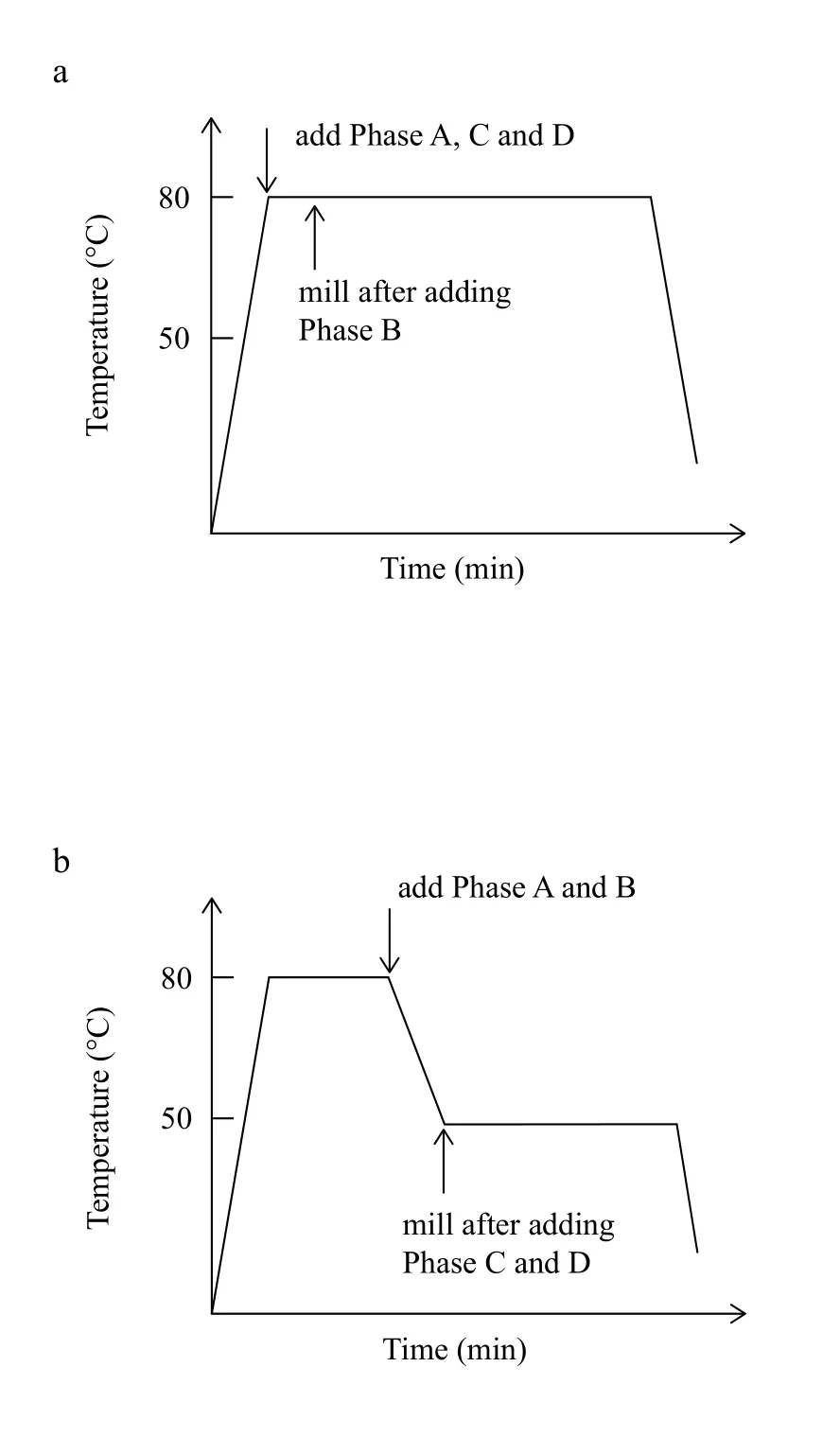
Figure 2. The schematic experimental routes

Preservative concentration determination instruments and methods
1.0 g of each sample was weighed accurately in a colorimetric tube. Then it was diluted with the mobile phase to 50 mL. After being diluted, these samples were dispersed by ultrasonication (30 min). Finally, they were be injected into micro vial by syringe filters to be ready for test. A Waters 2690 series high-performance liquid chromatography(HPLC) unit with a Waters 2487 Dual λ Absorbance Detector (DAD) has been employed and optimized to detect concentration of PE and p-HAP. For the separation,ZORBAX SB-C18 (250×4.6 mm, 5 μm) C18 column at room temperature was used. The mobile phase was composed of acetonitrile, methanol and 0.05 mol/L of NaH2PO4aqueous solution (adjusted to pH=3.5 with H3PO4) in a ratio of 10:30: 60 under isocratic conditions. The flow rate of the mobile phase was 1.0 mL/min, and injection volume was 10 μL. The analytes were detected by DAD at 280 nm.
Result and discussion
As a new preservative system, the relative content of both CPEand Cp-HAPwill be explored in several oil-water phases.Besides, different control factors from both formulation and processing angles will also be investigated to reveal their effects on CPEand Cp-HAP.
Definition of relative content
Relative content is defined as:

In equation 1, variable cpis defined as the concentration of preservative in water phase, which could be directly integrated from HPLC data. Another parameter cois defined as concentration of preservative in water phase if it is fully dissolved in water phase, which is constant. In this communication, both values of cofor PE and p-HAP equal to 0.8% (Co= (0.4%/0.5%)×100%). Based on this definition, relative content could reflect the percentage of preservative in water phase. The change of relative content could show us whether the preservative will migrate out of water phase or not after series of processing.
CPE and Cp-HAP in different oil-water system
Generally, plenty of oils and fats are used in personal care product to provide many functions such as emollient,moisturizer, softener and smoother et al.. Herein, eight different typical oils were chosen to set up the different demulsification oil-water systems, while composition of water phase was kept the same. It should be explained that although cetostearyl alcohol has been reported to have the function of co-emulsifier,it was still treated as an emollient here. CPEand Cp-HAPare calculated based on Equation 1. Each result was presented in Table 2. It is clear that values of CPEin squalane, petrolatum,silicone oil and hydrogenated polyisobutene system are 81%,84%, 90% and 84%. And values of Cp-HAPare 95%, 98%, 99%and 91%, respectively. Based on these data, concentrations of both PE and p-HAP are almost unchanged compared with its original concentration, which reflect preservatives are mainly located in water phase. Meanwhile, it is found that values of CPEin GTCC and cetostearyl alcohol are 25% and 28%, which respectively denoted about 75% of PE was not in water phase.Values of Cp-HAPalso exhibit the similar trend in both GTCC and cetostearyl alcohol systems. Both of them equal to 35%. Isononylisononanoate and olive oil are quite different against other oil phase. Both of them show an opposite trend. Values of CPEare 36% and 39%, while values of Cp-HAPequal to 51% and 63%. It reveals about 64% of PE and 49% of p-HAP has migrated out of water phase in isononyl isononanoate-water system. And 61% of PE and 37% of p-HAP are “wasted” approximately in olive oil-water system. GTCC, olive oil, cetostearyl alcohol and isononyl isononanoate are commonly used oil in cosmetic product. However, these data show that most of preservatives are not dissolved in water phase to play the role of preservation.According to the positive data from squalane, petrolatum,silicone oil and hydrogenated polyisobutene, it can be concluded these four oils might be good candidates for formulation when using PE and p-HAP preservative system.
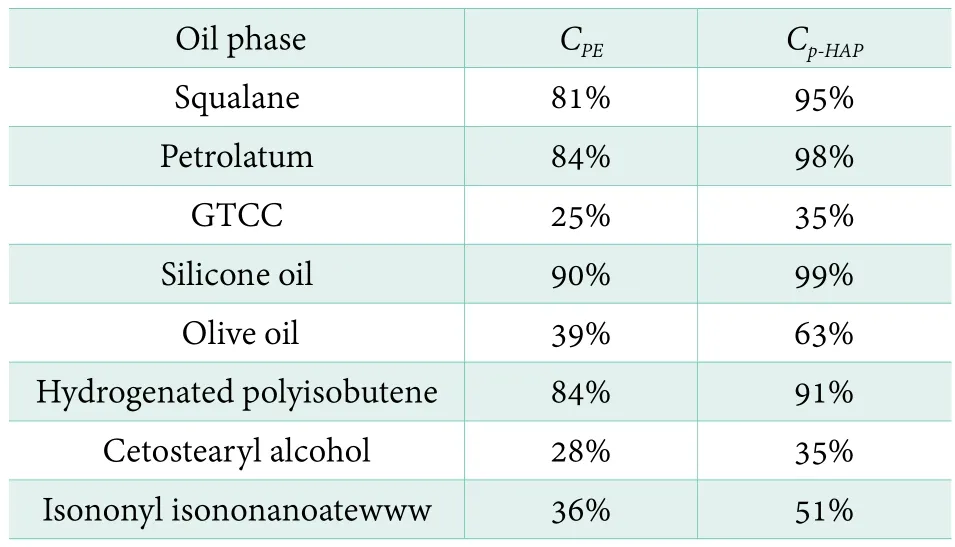
Table 2. Calculated CPE and Cp-HAP values in differentoil-water system (10% of BG)
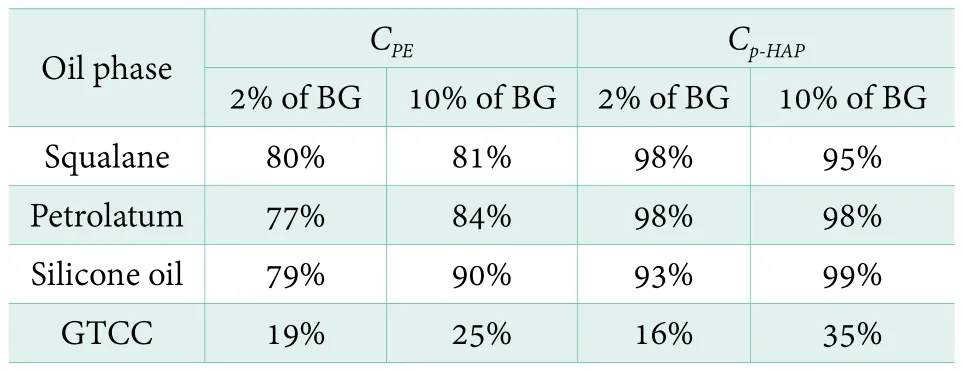
Table 3. CPE and Cp-HAP values in four oil phase with different dosage of BG
Polyols effects on CPE and Cp-HAP
By migrating into the oil phase of a formulation, there might not be a sufficient concentration of the preservative in the water phase where micro-organisms are located to protect the formulation. Both PE and p-HAP seem to prefer distributing in GTCC phase. Will solubilizer break this regular? Polyols are frequently-used ingredients as humectants in all kinds of formulations. Actually, they can also play the role of solubilizer due to their amphiphilic structure. Herein, the dosage of BG was controlled to study the polyols effects on CPEand Cp-HAP. Table 3 shows that value of CPEis 19% in GTCC system while adding 2% of BG. When increase the percentage of BG to 10%,the value of CPEincreases to 25%. In the same condition,value of Cp-HAPalso increases from 16% to 35%. In other word, the results mean that 6% of PE and 19% of p-HAP could be additionally dissolved in water phase to have the chance to play the role of preservative at least. Most of the data show similar trend that both CPEand Cp-HAPdecrease with the increase of BG percentage. It indicates that adding BG could help PE and p-HAP to migrate into water phase.
Processing parameter effects on CPE and Cp-HAP
No matter in the model system we used or in real emulsification, processing is the key stage to form micro structure of oil/water phase. Mixing will provide extra energy to active oil and water phase to meta-stable state,which create more oil/water interface. Temperature may control molecular movement speed. Processing route could also bring some influence. Migration of preservatives from water phase to oil phase will definitely through these micro structures created by processing.Thus mixing energy, temperature and processing route,these three factors are further studied and discussed.
Mixing time effects.Generally, different mixing time,speed and blender could be applied to control external filed energy. With a view of the real emulsion formula processing, homogenizer is always employed to provide stronger shear energy. In this way, smaller oil droplets could be obtained to improve the formula stability and also realize other benefits. Therefore, homogenizer was used to mix oil and water phase in this work. Another variable parameter (homogenization speed) was also fixed to 3,000 rpm, which could be closer to real productivity.The only variant is homogenization time. It was adjusted to control external field energy, which are 5, 10, 20 and 30 min, respectively. Related values of Cp-HAPand CPEare calculated and plotted in Figure 3. The curves show that both CPEand Cp-HAPare inversely proportional to homogenization time.

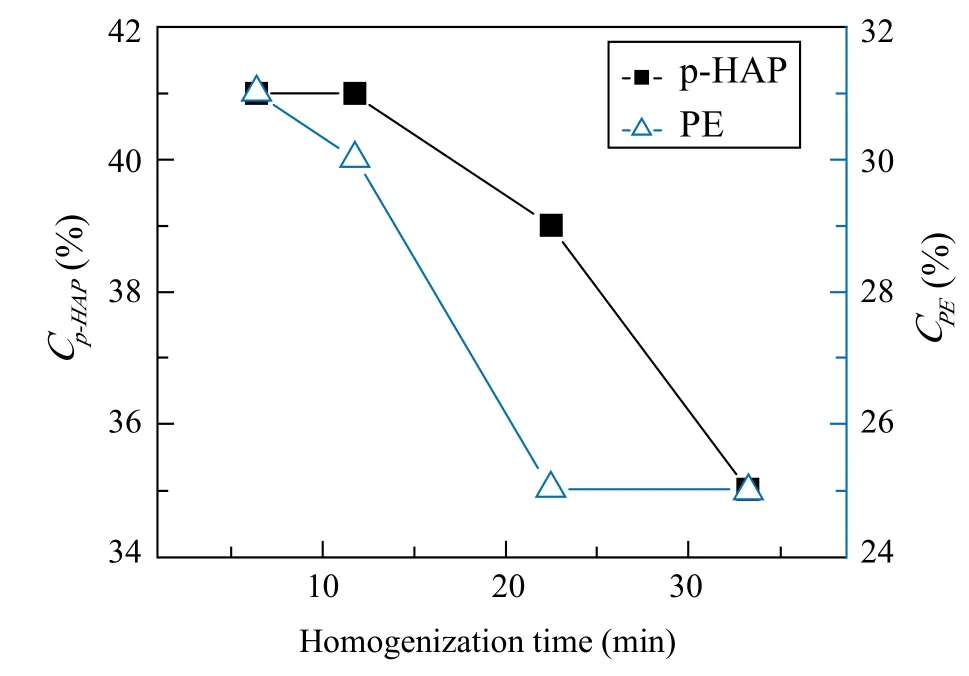
Figure 3. Cp-HAP and CPE against homogenization time
Temperature and processing route effects.Temperature and processing route factors are also considered together.Herein temperature is referring the temperature point where PE and p-HAP were adding into the system. The processing schematic route has been described in experimental section.It is noted that both values of CPEand Cp-HAPare sharply increased in cetostearyl alcohol phase when dropping the homogenization temperature from 80oC to 50oC. And in GTCC system, the data reveal the similar regulation. CPEand Cp-HAPwere very slightly increased when mixed under lower temperature. However, in isononyl isononanoate system,there is no significant difference.
GTCC, isononyl isononanoate and cetostearyl alcohol show the same trend that they could dissolve a majority of PE and p-HAP in oil phase when the system was homogenized at 80oC. When the mixture is milled under a lower temperature, cetrostearyl alcohol system shows a quiet different result compared with GTCC and isononyl isononanoate. Why does this occur?At the temperature of 50oC, the GTCC and isononyl isononanoate are both in the liquid state. It is much higher than their crystallization temperature. Regarding to cetostearyl alcohol, it tends to start crystallization at the temperature of 50oC. It is possible that migration rate of both PE and p-HAP are slower than the situation of 80oC. Although homogenization created more specific surface area between oil and water, the molecular diffusion rate might be limited by the solid interface.The mechanism is still under research. However, this interesting result provides us a new view to prevent PE and p-HAP to migrate into oil phase (Figure 4).
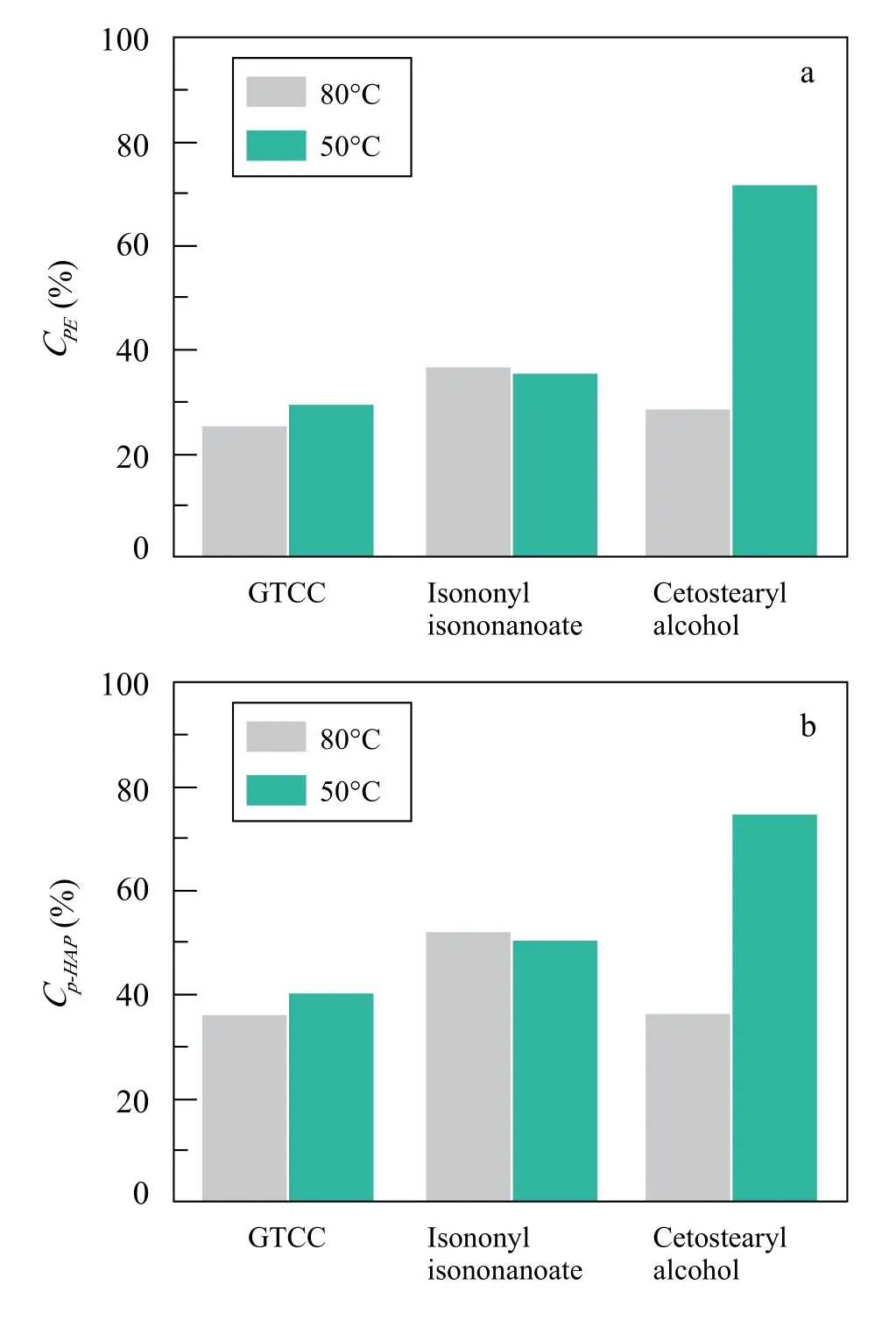
Figure 4. Bar graph of the calculated CPE and Cp-HAP values under different temperatures in GTCC, isononyl
Conclusion
In summary, this work has investigated the relative content of a novel preservative system (PE and p-HAP).Research data from eight frequently used oils show CPEand Cp-HAPare relatively lower in GTCC and cetostearyl alcohol. This indicates more than 72% of PE and 65%of p-HAP are migrating out of water phase in this simplified ideal O/W model. GTCC system has been further selected to study how to increase the CPEand Cp-HAP.Results reveal that increasing polyols dosage from 2%to 10% could increase 6% of PE and 19% of p-HAP to locate in water phase. Besides, it is found that processing parameters could also affect CPEand Cp-HAP. Based on all of these results, CPEand Cp-HAPcould be increased through below four approaches: (I) select oils which has a higher CPEand Cp-HAPor which could crystallize at higher temperature (above 50oC); (II) increase percentage of BG; (III) shorten homogenization time if possible; (IV) add preservatives at the end of process at lower temperature.
[1] Campana R.; Scesa C.; Patrone V.; et al. Microbiological Study of Cosmetic Products During Their Use by Consumers. Health Risk and Efficacy of Preservative Systems. Letters in Applied Microbiology 2006(43), 301-306.
[2] Yuancheng Hong; Friis A.; Leth T. Partition of Selected Food Preservatives in Fish Oil–Water Systems. Food Chemistry 2010(122), 60-64.
[3] Darbre P. D., Aljarrah A.; Miller W. R.; et al. Concentrations of Parabens in Human Breast Tumours. J. Appl. Toxicol 2004(24), 5-13.
[4] Fang B.; Yu M.; Zhang W.; et al. A New Alternative to Cosmetics Preservation and the Effect of the Particle Size of the Emulsion Droplets on Preservation Efficacy. International Journal of Cosmetic Science 2016(5), 496-503.
[5] Varvaresou A.; Papageorgiou S.; Tsirivas E.; et al. Self-Preserving Cosmetics. International Journal of Cosmetic Science 2009(31),163-175.
[6] Thyssen J. P.; Engkilde K.; Michael D. L.; et al. Temporal Trends of Preservative Allergy in Denmark. Contact Dermatitis 2010(62), 102-108.
[7] Waranyaboonchai R. Trend of Contact Allergy to Cosmetic Ingredients in Thai over a Period of 10 Years. Contact Dermatitis 2011(65), 311-316.[8] Ritatravassos A.; Claes L.; Boey L.; et al. Non-fragrance Allergens in Specific Cosmetic Products. Contact Dermatitis 2011(65), 276-285.
[9] Davies R.; M. B.; Johnston G.; et al. New and Emerging Cosmetic Allergens. Clinics in Dermatology 2011(29), 311-315.
[10] Lundov M. D.; Lise M.; Zachariae C.; et al. Contamination Versus Preservation of Cosmetics: A Review on Legislation,Usage, Infections, and Contact Allergy. Contact Dermatitis 2009(60), 70-78.
[11] Muyima N. O.; Zulu G.; Bhengu T.; et al. The Potential Application of Some Novel Essential Oils as Natural Cosmetic Preservatives in an Aqueous Cream Formulation. Flavour Fragr. J 2002(17), 258-266.
[12] Papageorgiou S.; Varvaresou A.; Tsirivas E.; et al. New Alternatives to Preservation. Cosmet. Sci 2010(61), 107-123.
[13] Jacobs M. G.; Henry S. M.; Cotty V. F. The Influence of pH,Emulsifier, and Accelerated Ageing upon Preservative Requirements of O/W Emulsion. J. Soc. Cosmet. CHEM, 1973(26):105-117.
[14] Toler J. C. Preservative Stability and Preservative Systems.International Journal of Cosmetic Science 1985(7), 157-164.
[15] Watrobska-swietlikowska D.; Malgorzata S. Partitioning of Parabens between Phases of Submicron Emulsions Stabilized with Egg Lecithin. International Journal of Pharmaceutics 2006(312),174-178.
 China Detergent & Cosmetics2016年4期
China Detergent & Cosmetics2016年4期
- China Detergent & Cosmetics的其它文章
- Preparation Technology and Property Research of Ultra-Concentrated Liquid Detergent
- Biodegradation of Nonylphenol Ethoxylates in the Continuous Flow Activated Sludge Simulation Test
- Alkoxylation for Surfactant Productions:towards the Continuous Reactors
- Development of Alternative to Animal Testing on Cosmetics by Middle-out Strategy
- Introduction on the Standardization and Future Work of Surfactant and Detergent in China
- National Standard of Laundry Powders (Phosphate Free)
