Bispectral index-guided fast track anesthesia by sevoflurane infusion combined with dexmedetomidine for intracranial aneurysm embolization: study protocol for a multi-center parallel randomized controlled trial
Chao-liang Tang, Juan Li, Bo Zhao Zhong-yuan Xia
1 Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Anesthesiology, Southern Branch of Anhui Provincial Hospital, Hefei, Anhui Province, China
BACKGROUND
An intracranial aneurysm is a local bulge or ballooning of an arterial wall in the brain that forms under arterial partial pressure to thin arteries in the brain. When an intracranial aneurysm ruptures, the result is a subarachnoid hemorrhage or intraparenchymal hemorrhage, accompanied by severe headache, vomiting, different levels of consciousness (Brisman et al., 2006).
Although surgical clipping was the first treatment introduced for intracranial aneurysms, in recent years,evidence has shown that interventional embolization is more effective (Brilstra et al., 1999; Raja et al., 2008). To embolize an intracranial aneurysm, a detachable balloon or a special electrolysis-spring microcatheter is inserted into the aneurysm (Xu, 2000). It is a highly promising method,characterized by not needing a craniotomy, and causing less trauma and less bleeding than currently available methods(Bai and Yang, 2007). However, during intracranial aneurysm embolization, elevated blood pressure or excessively decreased intracranial pressure that results from anesthesia can increase transmural pressure and stress to the blood vessel wall, increasing the risk of aneurysm rupture. Given this, careful selection of the anesthetic agent and anesthetic method is preferred.
Fast-track anesthetic techniques contribute to a rapid recovery from general anesthesia, which is important for timely processing of adverse events in patients (Ali Hassan,2015; Aniskevich and Pai, 2015). Despite the usefulness of these fast-track techniques, uniform standards for them are lacking. Fast-track anesthesia can be achieved using a balanced anesthesia technique and a combination of drugs given at the minimum dosage required to obtain the best effect. Because of the low demand for muscular relaxation,fast-track anesthesia is preferred for intracranial aneurysm embolization.
Sevoflurane is a commonly used anesthetic drug for aneurysm embolization. As a new, safe, and effective inhalable anesthetic, sevoflurane is characterized by rapid absorption/discharge and good controllability (Sakai et al., 2005),along with neuroprotective effects such as reducing brain metabolic rate and having little effect on cerebral blood flow. However, sevoflurane can also elevate intracranial pressure (Holmstr?m and Akeson, 2004) and trigger respiratory depression (Doi and Ikeda, 1987). Therefore, a low dose of sevoflurane can be used to reduce the likelihood of adverse reactions during an aneurysm embolization surgery.
Dexmedetomidine (DEX) is a novel, highly selective adrenoceptor agonist (Cormack et al., 2005) that produces dose-dependent sedative, anxiolytic, and analgesic effects,and also has anti-sympathetic and anti-shivering properties(Yuen et al., 2007). It exhibits mild effects on respiratory depression, strong intrinsic activity, has a short elimination half-life, and is non-addictive. Thus, DEX can be used for semi-arousable sedation and cooperative sedation, acting as an auxiliary analgesic to enhance sedative and analgesic effects, maintain hemodynamic stability, and reduce the necessary analgesic dosage of other analgesic agents(Paris and Tonner, 2005; Giovannitti et al., 2015). While DEX effects have been retrospectively examined in many studies (Table 1), its clinical applications as an auxiliary analgesic for sevoflurane anesthesia in intracranial aneurysm embolization have not been adequately investigated in prospective, parallel, randomized controlled trials. By retrieving ClinicalTrial.gov and Chinese Clinical Trial Register, we found that several study protocols are ongoing for prospective parallel randomized controlled trials using sevoflurane anesthesia combined with DEX in intracranial aneurysm embolization (Table 2).
Here, a randomized controlled clinical trial is designed to explore the use of rapid-track anesthesia with DEX combined with sevoflurane in intracranial aneurysm embolization by monitoring the bispectral index (BIS). We will attempt to realize a balanced anesthesia and rapid recovery in neurosurgical patients, which should help surgeons to treat adverse events in a timely manner.
METHODS/DESIGN
Study design
This will be a multi-center, parallel, randomized controlled trial, which will be conducted at Anhui Provincial Hospital and Renmin Hospital of Wuhan University, China. One hundred and twenty patients with intracranial aneurysm will be enrolled and randomly assigned to either a normal saline group or a DEX group (n = 60 per group). While monitoring the BIS, patients will be given intravenous infusion of 1.0 μg/kg DEX or normal saline for at least 10 minutes before anesthesia induction via propofol (see below), followed by continuous infusion of DEX (0.3 μg/kg per hour) or normal saline once anesthesia has been induced. Intraoperatively,anesthesia will be maintained with 2–3% sevoflurane in both groups.
BIS is mainly used to monitor the depth of anesthesia.BIS values have been shown to be highly associated with sedation, consciousness, and memory. The BIS index is a useful tool for monitoring normal physiological sleep and sedative components at different anesthetic depths (Bresil et al., 2013; Kim et al., 2014). The use of BIS monitoring helps to determine anesthesia depth, adjust amount of anesthetic agents administered, and reduce recovery time from general anesthesia (Bresil et al., 2013; Kim et al., 2014). Data from BIS monitoring will thus improve the anesthetic effect ofDEX infusion combined with sevoflurane in patients undergoing intracranial aneurysm embolization.
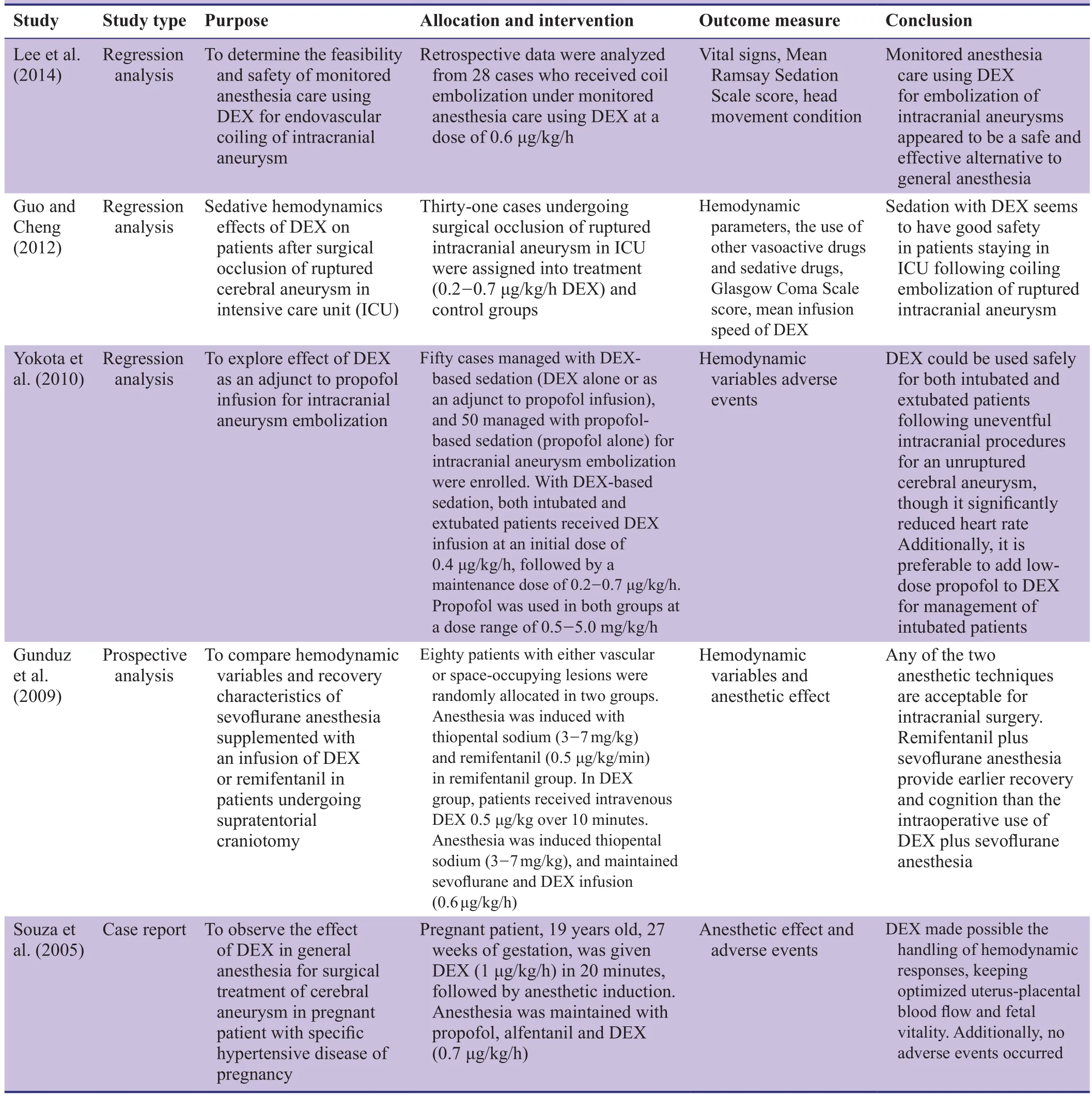
Table 1: International studies about dexmedetomidine (DEX) as an auxiliary analgesic
Anesthetic effects, postoperative recovery times from anesthesia, sevoflurane dosages, Ramsay Sedation Scale(RSS) scores, Visual Analogue Scale (VAS) scores, and BIS values will be compared between the two groups. Additionally, changes in physiological indicators and incidence of adverse events will be recorded. A flow chart of the trial protocol is shown in Figure 1.
Study participants
Patients undergoing intracranial aneurysm embolization will be recruited from Anhui Provincial Hospital and Renmin Hospital of Wuhan University, China.
Inclusion criteria
Patients must meet all the criteria below for recruitment in the trial:
· Diagnosed with intracranial aneurysm as shown bycomputed tomographic angiography/digital subtraction angiography
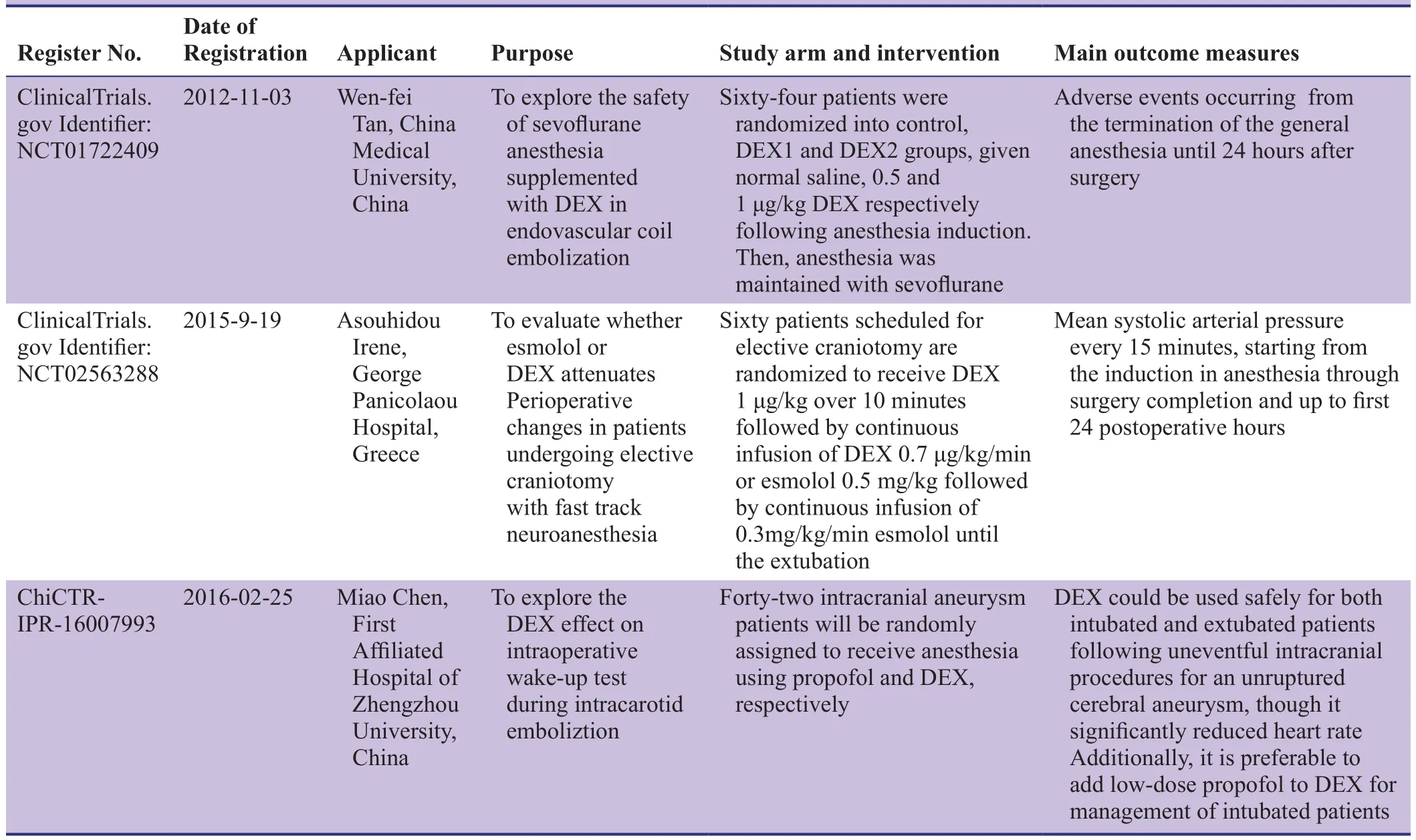
Table 2: Clinical trials using dexmedetomidine (DEX) as an adjunct to sevoflurane anesthesia in intracranial aneurysm embolization that are registered in ClinicalTrial.gov or the Chinese Clinical Trial Register
· Eligible for interventional therapy
· 18–70 years of age
· American Society of Anesthesiologists (ASA) physical status classification, grade I-IV (Fitz-Henry, 2011)
Exclusion criteria
Patients will be excluded from the trial if they meet any of the following criteria:
· Coagulation dysfunction
· Have previous history of drug allergy to DEX and sevoflurane
· Severe hypertension and cardiovascular disease
· Liver or kidney dysfunction
· Use of sedatives within 2 days before surgery
· Sinus bradycardia
Sample size
Time from the end of anesthesia until spontaneous breathing recovery will be recorded as main outcome measure for sample size calculation. As previously reported (Gu et al.,2013; Xu et al., 2013; Huang and Jiao, 2015), the time of recovering spontaneous breathing from sevoflurane anesthesia alone is about 6.5 minutes, while the recovery time from sevoflurane anesthesia with DEX infusion is expected to drop to 5.0 minutes, with a standard deviation of 2.3. If α = 0.05 and 1 - β = 0.9,= 41 (where tαand tβare the values corresponding to α and β, respectively; s is overall standard deviation for the two estimates; and δ is the mean difference in the two groups). Given a dropout rate of 20%, at least 50 patients will be needed per group. Funding for this trial allows us to recruit 60 patients per group.
Recruitment
Ongoing recruitment of patients will be carried out by attending physicians at Anhui Provincial Hospital and Renmin Hospital of Wuhan University. After written informed consent is given, patients will be screened using the inclusion and exclusion criteria.
Randomization
One hundred and twenty patients will be randomly numbered by statisticians according to the date of hospitalization,and will be evenly divided into a normal saline group(sevoflurane anesthesia with normal saline) and a DEX group (sevoflurane anesthesia with DEX). Evaluators will be unaware of study allocation.
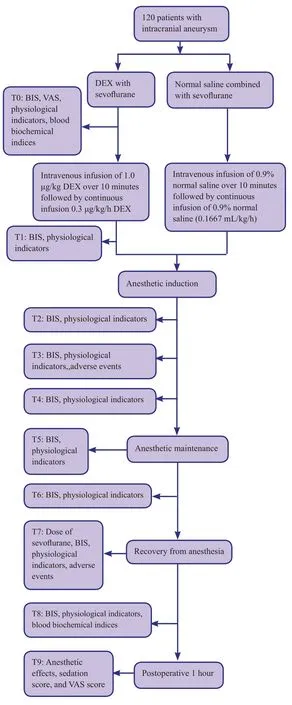
Figure 1: Flow chart of the trial.
Interventions
DEX group
1.0 μg/kg DEX (Sichuan Guorui Pharmaceutical Co., Ltd.,Leshan, Sichuan Province, China) will be formulated by 0.9% normal saline into 4 μg/mL, and infused intravenously within 10 minutes before anesthesia induction. Then, anesthesia will be maintained by continuous infusion of 0.166 mL/kg/h (0.3 μg/kg/h).
Normal saline group
Patients will be given intravenous infusion of 0.9% normal saline for 10 minutes before anesthesia induction, followed by continuous infusion of 0.9% sodium chloride at a flow rate of 0.166 mL/kg/h.
Intracranial aneurysm embolization
(1) Induction of anesthesia: patients will be intravenously given 1.5–2 mg/kg propofol (AstraZeneca, Italy) and 0.1–0.15 mg/kg cisatracurium (Shanghai Hengrui Pharmaceutical Co., Ltd.). After induction, an I-gel laryngeal mask airway (LMA) (Intersurgical Ltd., Wokingham, Berkshire, UK) will be inserted. LMA size will be determined according to patient weight: size # 3 for weights of 30–60 kg and #4 for weights of 50–90 kg. Successful LMA insertion will be defined as proper movement of the chest, a smooth end-tidal CO2tension wave without air leakage at an airway pressure > 20 cmH2O. Then, a gastric tube will be inserted via the LMA and connected to a Datex-Ohmeda S/5 Avance Anesthesia Machine (Datex-Ohmeda Inc., Madison, WI,USA) followed by mechanical ventilation at a capacity control mode (tidal volume: 6–8 mL/kg; frequency: 10–12 times/min). Intraoperative end-tidal CO2level will be set to a normal range of 35–45 mmHg.
(2) Maintenance of anesthesia: Intraoperative anesthesia will be maintained with 2–3% sevoflurane inhalation(Maruishi Pharmaceutical Co., Ltd., Osaka, Japan).
(3) Endovascular coiling of the aneurysm: Surgical treatment of aneurysm by endovascular coiling in all enrolled patients will be completed by the same surgeon. Using the Seldinger technique, a catheter will be inserted into the right femoral artery via a 6-ft-long braided introducer sheath and passed through the blood vessels into the aneurysm. A guglielmi detachable coil (ev 3 Inc.,Plymouth, MN, USA) will be inserted via the catheter,aided by cerebral angiography. The coil will be packed into the aneurysm to initiate a thrombotic reaction and block normal blood flow in the aneurysm. If successful,the sheath will be removed and a pressure bandage will be applied at the puncture site.
(4) Recovery from anesthesia: The evaporator will be closed at about 10 minutes before the end of surgery, and the flow rate of fresh gas will be reduced to 0.3–0.5 L/min.When the surgeon sutures the femoral artery using a vascular closure device (Abbott, Santa Clara, CA, USA),the flow rate of fresh gas will be increased to 4 L/min and anesthesia will be terminated.
(5) Postoperative management: Patients will be given routine treatments including lowering intracranial pressure, dehydration, and continuous intravenous infusion of 0.15% nimodipine (Bayer, Leverkusen, Germany) to prevent cerebral vasospasm.
Outcome measures
Primary outcomes
Primary outcomes will include anesthesia time, operative time, time from the end of anesthesia until recovering spontaneous breathing, time until eye opening on command, and time until the patient is oriented to time and place.
Secondary outcomes
· Dose of sevoflurane: The total amount of sevoflurane that is used during anesthesia maintenance will be recorded when the LMA is removed.
· RSS score: RSS scores will be determined at baseline and 1 hour after aneurysm coiling. RSSis a traditional method to assess the level of sedation at six different levels (Kress and Hall, 2006). Higher levels indicate greater sedation.
· VAS score: VAS scores for pain will be assessed at baseline and 1 hour after aneurysm coiling. The VAS is a scale to measure subjective sensations that has few influencing factors, and is widely used in clinical and basic research(Collins et al., 1997). When responding to a VAS item, a pastient will specify the level of pain by marking a point along a 100-mm horizontal line between two end-points that represent no pain and severest pain, respectively. A lower score indicates greater anesthesia.
· BIS value: BIS is a simple quantitative index that indicates whether the cerebral cortex is excited or depressed (Mitchell-Hines et al., 2016). The BIS value varies depending on sedation, consciousness, and memory. It is a preferred tool for monitoring sedative components at different anesthetic depths. It has a range from 0 to 100 (0, coma; 40–65, general anesthesia; 65–85, sedated state; 100, awake). If < 40, the patient is likely to present with outburst inhibition (Recart et al., 2003; Punjasawadwong et al., 2007).
Other outcomes
In this trial, the following additional outcomes will be recorded: physiological indexes including systolic blood pressure, diastolic blood pressure, and heart rate at different stages of anesthesia, blood biochemical parameters including levels of venous blood glucose, plasma neuron-specific enolase, S100β, interleukin-1β, 6, 8, and 10, tumor necrosis factor α, malondialdehyde, and superoxide dismutase before and after anesthesia. We will also record incidences of adverse events when inserting or removing the LMA, such as cough, restlessness, and chills.
Baseline data of patients will be listed in Table 3.
Flowchart of the trial process is shown in Table 4.
Table 3: Baseline data of patients
Data collection, management, analysis, and open access
· Data collection: All data from patients will be collected on paper and electronic case report forms. Only the persons who have permission will have the right to query the ResMan database for data entry, modification, and access. Others will be not allowed to query the database or access any clinical data. These measures will ensure the safety and confidentiality of the database.
· Data management: whenever any changes or corrections in the paper case-report form are recorded, the original records should be clearly retained. Additionally, the date of change/correction, signature, and a reason should be noted. Any data entry, including alterations, deletions,and additions, will be recorded by an electronic data capture system beginning at the first data entry. Track inspectors should be set to be protected by this system,and no artificial modification or editing will be allowed.Complete rights management must be established for the ResMan database, and only authorized personnel will be allowed to query the database.
· Data analysis: All data will be statistically analyzed by professional statisticians. An independent data monitoring committee will be responsible for data monitoring and management throughout the entire trial to ensure scientific accuracy, stringency, authenticity, and integrity.
· Open data: Anonymized published data will be released at http://www.figshare.com.

Table 4: Schedule for outcome measurement
Statistical analysis
All data will be statistically analyzed by statisticians using the SPSS 23.0 statistical software package (IBM Corp., Armonk, NY, USA) in line with the intention-to-treat principle.All measurement indexes will be expressed as the mean ±SD, except for incidence of adverse events, followed by a normal distribution analysis. Normally distributed data will be compared using independent samples t-test, and non-normally distributed data compared using the Wilcoxon test. Chi-squared tests will be used to compare incidences of adverse events between groups. A value of P < 0.05 will be considered statistically significant.
Adverse events
During the trial, all serious adverse events will be recorded in detail, including the date of occurrence, type of event,onset duration, symptoms, time of drug withdrawal, treatments, and outcomes, all of which will be reported to the responsible department, bid unit, ethics committee, drug regulatory department, and any other relevant administrative departments within 24 hours. Once any adverse events occur, the patient will be given corresponding therapeutic measures and drugs will be withdrawn in a timely manner.
Auditing
Trial progress will be reported to the ethics committee of Anhui Provincial Hospital and Renmin Hospital of Wuhan University every 6 months or every year. Between reports,relevant data will be updated in the registration database.
Confidentiality
The research group and the medical institution to which it belongs will have the right to use all data from the trial without compensation. Patient information will be encrypted and stored by the Anhui Provincial Hospital and Renmin Hospital of Wuhan University. Ethics committee of the Anhui Provincial Hospital and Renmin Hospital of Wuhan University, drug regulatory department, and trial executors will have free access to patient information, but will not be allowed to disclose these data. Unless required by law,patient identity will not be disclosed. Findings of this trial will be published without revealing the identity of patients.
Data acquisition
All research data related to this clinical trial will belong to the Anhui Provincial Hospital.
TRIAL STATUS
Recruiting at the time of submission.
DISCUSSION
During intracranial aneurysm embolization, the anesthesi-ologist should help the patient maintain an appropriate level of sedation and analgesia, ensuring stable vital signs and systemic heparin administration. For an anesthesiologist,understanding all the risks and hazards of an intracranial aneurysm that can occur at different points in the procedure and maintaining close cooperation with the surgeon responsible for neurovascular intervention comprise the basis of intraoperative management. The purpose of this management is to prevent potentially fatal ischemia and bleeding. A rapid recovery from anesthesia assists the surgeon because the nervous system function can be assessed as early as possible.
DEX as an adjunct drug for general anesthesia and sedation can shorten the time until awakening and extubation,stabilize hemodynamic parameters, and reduce adverse events. Thus, DEX is currently a well-regarded and popular drug. As previously reported (Gu et al., 2013; Xu et al.,2013; Huang and Jiao, 2015), this trial will use DEX as an anesthetic supplement to sevoflurane anesthesia in patients undergoing endovascular aneurysm coiling. The authors will provide objective evaluation of DEX anesthesia. However, because of differences in the etiology, disease course,and sex of the patients recruited in the trial, future research will need to focus on these potential confounding factors.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms. In the form the patient(s) will give his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
None declared.
Author contributions
CLT and ZYX designed this trial protocol; and JL and BZ will collect data. All authors read and approved the final version of this manuscript for publication.
Plagiarism check
This manuscript was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
Ali Hassan HI (2015) Comparison between two different selective spinal anesthesia techniques in ambulatory knee arthroscopy as fast-track anesthesia. Anesth Essays Res 9:21-27.
Aniskevich S, Pai SL (2015) Fast track anesthesia for liver transplantation: review of the current practice. World J Hepatol 7:2303-2308.
Bai JJ, Yang RG (2007) The experience of the anesthesia of the embolization for intracranial aneurysm through DSA. Jiceng Yixue Luntan 11:398-399.
Bresil P, Nielsson MS, Malver LP, Kraemer K, Schj?Rring O, Dethlefsen C, Lambert PH (2013) Impact of bispectral index for monitoring propofol remifentanil anaesthesia. A randomised clinical trial. Acta Anaesthesiol Scand 57:978-987.
Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A(1999) Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke 30:470-476.
Brisman JL, Song JK, Newell DW (2006) Cerebral aneurysms. New Engl J Med 355:928-939.
Collins SL, Moore RA, McQuay HJ (1997) The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain 72:95-97.
Cormack JR, Orme RM, Costello TG (2005) The role of alpha2-agonists in neurosurgery. J Clin Neurosci 12:375-378.
Doi M, Ikeda K (1987) Postanesthetic respiratory depression in humans: a comparison of sevoflurane, isoflurane and halothane. J Anesth 1:137-142.
Fitz-Henry J (2011) The ASA classification and peri-operative risk.Ann R Coll Surg Engl 93:185-187.
Giovannitti JA, Thoms SM, Crawford JJ (2015) Alpha-2 adrenergic receptor agonists: a review of current clinical applications.Anesth Prog 62:31-38.
Gu JF, Li WH, Fu J, Ran R, Tian G (2013) Effect of dexmedetomidine on the patients with intracranial aneurysm embolization at perioperative period. Mazui yu Zhentong 10:85-87.
Gunduz M, Gunes Y, Ozbek H, Yilmaz D, Isik G (2009) Comparison of dexmedetomidine or remifentanil infusion combined with sevoflurane anesthesia in craniotomy: hemodynamic variables and recovery. Neurosurg Q 19:116-119.
Guo R, Cheng R (2012) Evaluation of the sedative effect of dexmedetomidine on patients after surgical occlusion of ruptured cerebral aneurysm in intensive care unit. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 24:306-308.
Holmstr?m A, Akeson J (2004) Desflurane increases intracranial pressure more and sevoflurane less than isoflurane in pigs subjected to intracranial hypertension. J Neurosurg Anesthesiol 16:136-143.
Huang CF, Jiao F (2015) Dexmedetomidine combined with sevoflurane inhalation anesthesia in intracranial aneurysm embolization.Jiangxi Yiyao 50:355-357.
Kim JK, Kim DK, Lee MJ (2014) Relationship of bispectral index to minimum alveolar concentration during isoflurane, sevoflurane or desflurane anaesthesia. J Int Med Res 42:130-137.
Kress JP, Hall JB (2006) Sedation in the mechanically ventilated patient. Crit Care Med 34:2541-2546.
Lee HH, Jung YJ, Choi BY, Chang CH (2014) Usefulness of Dexmedetomidine during Intracerebral Aneurysm Coiling. J Korean Neurosurg Soc 55:185-189.
Mitchell-Hines T, Ellison K, Willis S (2016) Using bispectral index monitoring to gauge depth of sedation/analgesia. Nursing 46:60-63.
Paris A, Tonner PH (2005) Dexmedetomidine in anaesthesia. Curr Opin Anaesthesiol 18:412-418.
Punjasawadwong Y, Boonjeungmonkol N, Phongchiewboon A(2007) Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev:CD003843.
Raja PV, Huang J, Germanwala AV, Gailloud P, Murphy KP, Tamargo RJ (2008) Microsurgical clipping and endovascular coiling of intracranial aneurysms: a critical review of the literature. Neurosurgery 62:1187-1202; discussion 1202-1183.
Recart A, Gasanova I, White PF, Thomas T, Ogunnaike B, Hamza M, Wang A (2003) The effect of cerebral monitoring on recovery after general anesthesia: a comparison of the auditory evoked potential and bispectral index devices with standard clinical practice. Anesth Analg 97:1667-1674.
Sakai EM, Connolly LA, Klauck JA (2005) Inhalation anesthesiology and volatile liquid anesthetics: focus on isoflurane, desflurane,and sevoflurane. Pharmacotherapy 25:1773-1788.
Souza KM, Anzoategui LC, Pedroso WC, Gemperli WA (2005)Dexmedetomidine in general anesthesia for surgical treatment of cerebral aneurysm in pregnant patient with specific hypertensive disease of pregnancy: case report. Rev Bras Anestesiol 55:212-216.
Xu DC (2000) Applied anatomy of clinical bone defect repair. Beijing, China: China Medical Science Press.
Xu YY, Wu DS, Zheng ZY (2013) Dexmedetomidine combined with sevoflurane controlled hypotension in the endovascular treatment of intracranial aneurysms. Yixue Xinxi 26:262-263.
Yokota H, Yokoyama K, Noguchi H, Nishioka T, Umegaki O, Komatsu H, Sakaki T (2010) Post-operative dexmedetomidine-based sedation after uneventful intracranial surgery for unruptured cerebral aneurysm: comparison with propofol-based sedation. Neurocrit Care 14:182-187.
Yuen VM, Irwin MG, Hui TW, Yuen MK, Lee LH (2007) A doubleblind, crossover assessment of the sedative and analgesic effects of intranasal dexmedetomidine. Anesth Analg 105:374-380.
 Asia Pacific Journal of Clinical Trials:Nervous System Diseases2016年4期
Asia Pacific Journal of Clinical Trials:Nervous System Diseases2016年4期
- Asia Pacific Journal of Clinical Trials:Nervous System Diseases的其它文章
- Utilization patterns of benzodiazepines in psychiatric patients in a tertiary care teaching hospital
- Homeopathic prophylaxis for recurrent urinary tract infections following spinal cord injury: study protocol for a randomized controlled trial
- Effectiveness of cerebellar repetitive transcranial magnetic stimulation in essential tremor: study protocol for a randomized, sham-controlled trial
- Cognitive function and biomarkers after traumatic brain injury:protocol for a prospective inception cohort study
- Electroencephalographic changes following trigeminal nerve stimulation for major depressive disorder: study protocol for a randomized sham-controlled trial
- Effect of intravenous acetaminophen on post-operative opioidrelated complications: study protocol for a randomized,placebo-controlled trial
