Synthesis and Properties of Dendritic Long-Chain Esters as Crude Oil Flow Improver Additives
(Provincial Key Laboratory of Oil Gas Chemical Technology, College of Chemistry Chemical Engineering, Northeast Petroleum University, Daqing 163318)
Synthesis and Properties of Dendritic Long-Chain Esters as Crude Oil Flow Improver Additives
Li Cuiqin; Sun Peng; Shi Weiguang; Wang Jun
(Provincial Key Laboratory of Oil Gas Chemical Technology, College of Chemistry Chemical Engineering, Northeast Petroleum University, Daqing 163318)
The ef fi ciencies of 6 kinds of macromolecules with dendritic structure in improving the fl ow properties of crude oil were investigated. The dendritic additives were synthesized using low-generation dendritic poly(amidoamine) and alkyl longchain acrylic esters as starting materials, and their structures were characterized by the Fourier transform infrared spectroscopy,1H-nuclear magnetic resonance and elemental analysis. The effects on the pour point and rheological properties of crude oil samples were studied. Ef fi ciencies of dendritic long-chain esters were not only in fl uenced by the alky chain length, but also by the generation of dendrimer. The longer the alkyl chain of dendritic long-chain ester was, the better the effect in the reduction of pour point and apparent viscosity was. Ef fi ciencies of 1.5 generation dendritic long-chain ester with 8 branched chains for the reduction of pour point and apparent viscosity were superior to those of 0.5 generation dendritic long-chain ester with 4 branched chains. Under the same conditions, ef fi ciencies of 1.5 generation dendritic eighteen ester were superior to those of other 1.5 generation dendritic long-chain esters for the reduction of pour point and viscosity of crude oil.
dendrimer; fl ow improver; dendritic long-chain ester; pour point depressant
1 Introduction
Crude oil is one of the most actively traded commodities in the world, and global demand for crude oil has been maintaining a stable annual growth rate[1]. However, transportation of crude oil through pipelines is difficult due to its poor low mobility and fl owability. One of the major difficulties in the pipeline transportation is the highly viscous fl uid that requires ef fi cient and economical ways to transfer the crude oil.
Different methods are used in order to modify the fl uidity of the crude oil suitable for pipeline transportation. Currently, the methods for reducing the viscosity of crude oil to improve the fl ow properties are as follows: dilution with lighter crudes or alcohols, formation of an oil in water emulsion with surfactants, increasing or conserving oil temperature, and reducing crude oil’s pour point or viscosity by using crude oil fl ow modi fi ers[2]. Dilution methods could modify the mobility of crude oil in the process of transportation and avoid high pressure drop or the need for high temperature, but it may require substantial investment in pumping and pipeline transmission due to the increase of the transport volume and the needs to separate
the solvent at some point, in order to re-process the crude oil and subsequently return it to the oil production site. Heating technique could overcome the above mentioned problems related with dilution of transporting heavy oil by pipeline. However, the capital and operating costs will be signi fi cantly high especially during pipeline transportation of crude through a long distance[3]. The emulsion method could achieve a reduction in the apparent viscosity. But the major difficulty related with the use of this technology is the selection and cost of the surfactant component of the emulsion. The surfactant must be capable of stabilizing the emulsion during transportation, and it also must be capable of being subject to easy separation at the destination of the pipeline transport[4-5].
Another alternative to reduce heavy oil viscosity is the use of flow improvers that can affect nucleation, adsorption or solubility of waxes. The modi fi cation of wax crystallization may help to depress the pour point and viscosityof crude oil and yield stress appreciably. Many polymeric compounds have been described as flow improvers, which are chemical additives that can affect nucleation, adsorption or solubility of waxes. Various types of fl ow improvers that are extensively used for crude oil are polymers of n-alkyl acrylates and methacrylates with α-ole fi ns or long chain alcohols[6-7], polyvinyl ethers, in particular copolymers of olefins and vinyl esters[8], ethylene vinyl acetate (EVA) copolymers[9], copolymers of maleic anhydride with styrene or α-ole fi ns or vinylic esters, polyα-olefins and poly-n-alkyl acrylates and methacrylate copolymers[10]. The ethylene-vinyl acetate (EVA) copolymer is one of the most widely used cold fl ow improvers and has good activity in improving the fl ow properties of oils. However, the preparation of ethylene-vinyl acetate copolymer needs a high-pressure reaction condition and expensive equipment. Therefore, great efforts have been made to develop other ef fi cient cold fl ow improvers.
Dendrimer is a novel macromolecular compound with special structure which provides dendrimer with unique properties such as high degree of branching, globular architecture and well-defined molecular weight[11]. Dendrimers have shown bright application prospects in drug delivery, oilfield chemistry, host-guest chemistry, catalyst preparation, etc.[12-14]Dendritic poly(amidoamine) (PAMAM) and dendritic polyether serving as the demulsifiers are widely used to demulsify the crude oil emulsion. Furthermore, it is well known that polymers with branched structures can reduce the viscosity of organic compounds, and these polymers are used in many highquality engine oils[15].
In this paper, 6 kinds of dendritic long–chain esters with dendritic branched structure were synthesized according to the property of function transformation of dendritic PAMAM. These dendritic long-chain esters have different alkyl chain length and branched chain number. The quality of synthetic dendrimers functioning as pour point depressants and rheology improvers were tested on crude oil samples obtained from Daqing oil fi eld. The in fl uence of the structure of dendrimers used as crude oil fl ow improver additives on the pour point and rheological properties of crude oil samples was studied.
2 Experimental
2.1 Materials
Ethylamine, acrylic acid and cyclohexane were analytical reagents purchased from the First Shanghai Reagent Co. of China.p-Toluene sulphonic acid, 1-dodecanol, 1-octadecanol and hydroquinone were chemically pure reagents purchased from the Tianjin Kermel Chemical Reagent Development Center (China). A series of 1.0 generation dendritic PAMAM (1.0G PAMAM) compounds were synthesized in our laboratory[16]. All reagents were used as received without further puri fi cation. Crude oil was kindly supplied by the Daqing Oil fi eld Company Limited (China) and the physical parameters of crude oil at a temperature of 25 ℃ and a shear rate of 60 s-1are listed in Table 1.
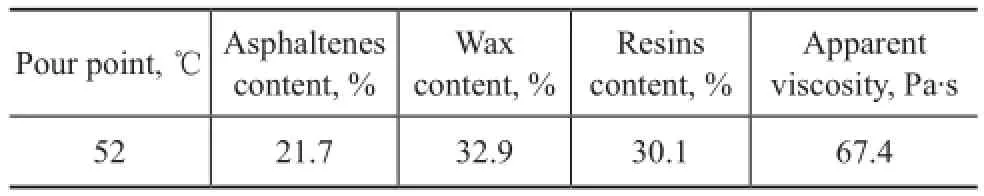
Table 1 Physical parameters of crude oil
2.2 Synthesis of long-chain alkyl acrylic esters
1-Dodecanol (40.0 g, 0.22 mol) was dissolved in 25 mL of cyclohexane at 40 ℃, and then hydroquinone (0.574 g, 0.005 mol) used as the inhibitor, p-toluene sulphonic acid (0.82 g, 0.005mol) used as the catalyst, and acrylic acid (103.2 g, 1.20 mol) were added into the flask equipped with a magnetic stirrer and a water divider. The mixture was stirred and re fl uxed for 8 h at 90 ℃. The solvent was removed by rotary evaporation until a white-yellow liquid was formed. The liquid was washed with a 5 % NaOH solution until the aqueous phase was colorless. Then it was rinsed with water in order to remove residues of the washing solution. The organic phase was then dried under vacuum for 12 h at 50 ℃ to give dodecyl acrylate with a yield equating to 96%. Analysis of the product was as follows: Found: C=74.9%; H=12.2%; and O=13.2%; Calculated: C=75.0%; H=11.7%; and O=13.3%.
The same method using n-dodecyl acrylate was intended to synthesize octadecyl acrylate and octyl acrylate. The yield of octadecyl acrylate was 92.7 %. Analysis of the product was as follows: Found: C=82.7%; H=9.6%; and O=7.7%; Calculated: C=82.9%; H=9.5%; and O=7.6%. The yield of octyl acrylate was 97.5%. Analysis of the productwas as follows: Found: C=71.7%; H=10.9%; and O=17.4%; Calculated. : C=71.9%; H= 10.6%; and O= 17.5%.
2.3 Synthesis of dendritic long-chain esters
2.3.1 Synthesis of 0.5 G dendritic long-chain esters
Ethylamine (1.2 g, 0.02 mol) was dissolved in 40 mL of methanol at 40 ℃. Dodecyl acrylate (28.8 g, 0.12 mol) was added dropwise for a period of 30 min. The mixture was stirred for 48 h at 40 ℃. When the reaction was over, the mixture was standing for 24 h at 25 ℃. The lower layer liquid was washed three times with 60 mL of methanol. Then it was rinsed with water in order to remove residues of the washing solution. The residual methanol was removed by rotary evaporation until a yellow liquid was obtained. The liquid was dissolved in 100 mL of acetone, and was allowed to stand for 48 h at 10 ℃. The white precipitate was collected by fi ltration and washed with acetone, and then dried under vacuum for 12 h at 30 ℃ to form the 0.5 generation dendritic twelve ester (0.5 G Dendrimer 12). The yield was 74.9 %. Analysis of the product was as follows: Found: C= 72.6%; H=12.0%; N=2.82%; and O=11.6%; Calculated: C=72.9%; H=11.8%; N=2.74%; and O=12.6%.1H NMR (CDCl3,δ): 2.62 (t, 4H, N-CH2-CH2-N), 2.88 (t, 8H, N-CH2-C-CO-), 2.56 (t, 8H, N-CCH2-CO-), 4.08 (m, 8H, O-CH2-C-), 1.82 (m, 8H, -(C)9-CH2-C), 1.46—1.51 (m, 72H, -O-C-(CH2)9-C-), 1.38 (t, 12H, -CH3).
The same method using 0.5 generation dendritic twelve ester was intended to synthesize the 0.5 generation dendritic eight ester (0.5G Dendrimer 8) and the 0.5 generation dendritic eighteen ester (0.5G Dendrimer 18). The yield of 0.5G Dendrimer 18 was 65.8 %. Analysis of the product was as follows: Found: C=73.7%; H=11.7%; N=2.48%; and O=12.1%; Calculated: C=73.9%; H=11.6%; N=2.54%; and O=12.0%.1H NMR (CDCl3,δ): 2.64 (t, 4H, N-CH2-CH2-N), 2.83 (t, 8H, N-CH2-C-CO-), 2.54 (t, 8H, N-C-CH2-CO-), 4.04 (m, 8H, O-CH2-C-), 1.80 (m, 8H, -(C)15-CH2-C), 1.40—1.50 (m, 72H, -O-C-(CH2)15-), 1.32 (t, 12H, -CH3).
The yield of 0.5G Dendrimer 8 was 82.6 %. Analysis of the product was as follows:-- Found: C=71.9%; H=11.7%; N=2.83%; and O=13.6%; Calculated: C=72.2%; H= 11.6%, N=2.90%; and O=13.3%.1H NMR (CDCl3,δ): 2.49 (t, 4H, N-CH2-CH2-N), 2.69 (t, 8H, N-CH2-C-CO-), 2.45 (t, 8H, N-C-CH2-CO-), 3.96 (m, 8H, O-CH2-C-), 1.62 (m, 8H, -(C)5-CH2-C), 1.37—1.42 (m, 40H, -O-C-(CH2)5-), 0.97 (t, 12H, -CH3).
2.3.2 Synthesis of 1.5 G dendritic long-chain esters
1.0 G PAMAM (5.16 g, 0.01 mol) was dissolved in 50 mL of methanol at 40 ℃. Dodecyl acrylate (28.8 g, 0.12 mol) was added dropwise in a period of 30 min. The mixture was stirred for 48 h at 40 ℃. When the reaction was over, the mixture was allowed to stand for 24 h at 25 ℃. The lower layer liquid was washed three times with methanol. Then it was rinsed with water in order to remove residues of the washing solution. The residual methanol was removed by rotary evaporation until a yellow liquid was formed. The liquid was dissolved in 50 mL of acetone, and the solution was allowed to stand for 48 h at 10 ℃. The white precipitate was collected by fi ltration and washed with acetone, and then dried under vacuum for 12 h at 30 ℃ to obtain the 1.5 generation dendritic twelve ester (1.5 G Dendrimer 12). The yield was 65.3%. Analysis of the product was as follows:-- Found: C=72.7%; H=11.0%; N=3.39%; and O=12.9%; Calculated: C=72.9%; H=11.1%; N=3.18%; and O=12.8%.1H NMR (CDCl3,δ): 2.90 (t, 4H, N-CH2-CH2-N), 2.78 (t, 24H, N-CH2-C-CO-), 3.08 (t, 24H, N-C-CH2-CO-), 5.86 (m, 4H, -C-C-CONH-), 3.48 (t, 8H, -C-CON-CH2-), 2.55 (t, 8H, -CON-C-CH2-N-), 4.05(t, 16H, -COO-CH2-C-), 1.88 (t, 16H, -(C)9-CH2-C), 1.55—1.62 (m, 144H, -O-C-(CH2)9-), 1.45 (t, 24H, -CH3).
The same method for synthesis of 1.5 G Dendrimer 12 was used to synthesize the 1.5 G dendritic eight ester (1.5G Dendrimer 8) and the 1.5 generation dendritic eighteen ester (1.5G Dendrimer 18). The yield of 1.5 G Dendrimer 18 was 57.9 %. Analysis of the product was as follows:--Found: C=75.7%; H=11.8; N=2.49%; and O=10.0%; Calculated: C=75.5%; H=11.7%; N=2.67%; and O=10.1%.1H NMR (CDCl3,δ): 2.88 (t, 4H, N-CH2-CH2-N), 2.82 (t, 24H, N-CH2-C-CO-), 3.02 (t, 24H, N-C-CH2-CO-), 5.79 (m, 4H, -C-C-CONH-), 3.44 (t, 8H, -C-CON-CH2-), 2.50 (t, 8H, -CON-C-CH2-N-), 4.02(t, 16H, -COO-CH2-C-), 1.85 (t, 16H, -(CH2)15-CH2-C), 1.51—1.69 (m, 240H, -OC-(CH2)15-), 1.43 (t, 24H, -CH3).
The yield of 1.5G Dendrimer 8 was 75.5 %. Analysis of the product was as follows: Found: C=70.0%; H=10.4%;N=3.98%; and O=15.6%; Calculated: C=69.8%; H= 10.3%; N=4.14%; and O=15.8%.1H NMR (CDCl3,δ): 2.92 (t, 4H, N-CH2-CH2-N), 2.80 (t, 24H, N-CH2-CCO-), 3.12 (t, 24H, N-C-CH2-CO-), 5.88 (m, 4H, -C-CCONH-), 3.49 (t, 8H, -C-CON-CH2-), 2.59 (t, 8H, -CONC-CH2-N-), 4.09(t, 16H, -COO-CH2-C-), 1.90 (t, 16H, -(C)5-CH2-C), 1.51—1.58 (m, 80H, -O-C-(CH2)5-), 1.50 (t, 24H, -CH3).
2.4 Sample preparation
Synthetic dendritic long-chain esters were dissolved in a definite amount of toluene. A certain volume of the toluene solution of dendritic long-chain ester was added gradually into a required amount of the crude oil under stirring, and the mixture was used to form stable and homogeneous sample by stirring. The sample was taken for measurements.
2.5 Experimental methods
The crude oil rheological measurements were carried out on a Brook fi eld viscometer at different temperatures and different shear rates. The range of temperature was from 25 ℃ to 65 ℃, and the shear rate ranged from 12 s-1to 168 s-1. The pour point of crude oil was measured according to the modified ASTM D 97-66 method[17]. The crude oil sample was fi rstly warmed (100 ℃) for 30 min to dissolve the precipitated wax, and then the sample was cooled down in a water bath.
3 Results and Discussion
3.1 Synthesis and characterization
Generally speaking, octyl acrylate homopolymer and copolymer are used to reduce the viscosity of the crude oil for pipeline transportation. 6 kinds of dendritic longchain esters with long-chain alkyl acrylic esters used as starting materials were designed and synthesized. To synthesize dendritic long-chain esters with high branched hydrophobic terminals, the long alkyl chain acrylic esters were used in excess in the reaction, denoting that there were unreacted long-chain alkyl acrylic esters left in the mixture after reaction. The unreacted long-chain alkyl acrylic ester was removed by washing the reacted mixture with lots of acetone. Dendritic long-chain esters with long-chain hydrophobic terminals were prepared according to Scheme 1.

Scheme 1 Routes for synthesis of dendritic long-chain esters
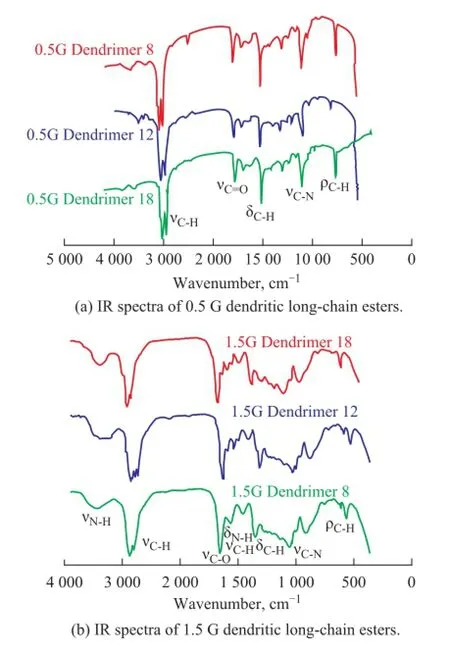
Figure 1 Infrared spectra of dendritic long-chain esters
Dendritic long-chain esters were characterized by elemental analysis, IR spectroscopy and1H-NMR. The IR spectra for the dendritic long-chain esters are shown in Figure 1. With respect to the 0.5 G dendrimers, IR spectra showed that the saturated C-H stretching vibration absorption peak appeared at 3 000—2 900 cm-1, and the C=O stretching vibration absorption peak appeared at 1 780 cm-1, while the C-H of -CH2- and -CH3deformation vibration absorption peak appeared at 1 510 cm-1, the C-N stretching vibration absorption peak appeared at 1 175 cm-1, and the C-H of -CH2- rocking vibration absorption peak appeared at 740 cm-1. As for the 1.5 G dendrimers, IR spectra showed that the N-H stretching vibration absorption peak appeared at 3 500—3 200 cm-1, and the saturated C-H stretching vibration absorption peak appeared at 3 000—2 900 cm-1, while the C=O stretching vibration absorption peak appeared at 1 780 cm-1, the N-H deformation vibration in coincidence with the C-N stretching vibration absorption peak appeared at 1 570—1 510 cm-1, and the C-H of -CH2- and -CH3deformation vibration absorption peak appeared at 1 490 cm-1, the C-N stretching vibration absorption peak appeared at 1 175 cm-1and the C-H of -CH2- rocking vibration absorption peak appeared at 740 cm-1. The results of elemental analysis and1H NMR spectroscopy (Figure 2) are summarized in the synthesis section. They agreed well with the FT-IR data to con fi rm the structure of the dendritic long-chain esters.
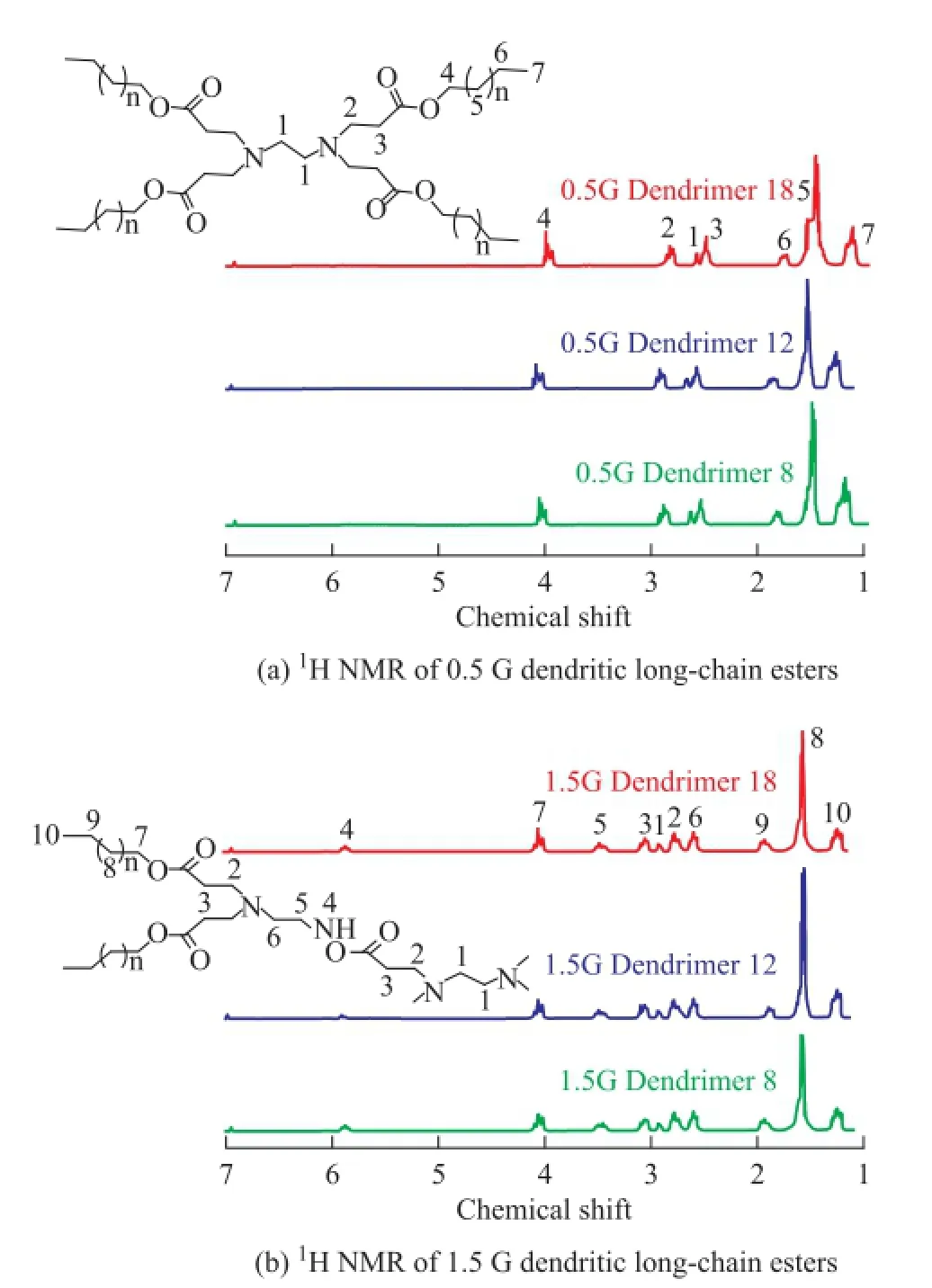
Figure 21H NMR of dendritic long-chain esters
3.2 In fl uence of temperature on viscosity of crude oil
Heating could enhance the flowability of crude oil and decrease the viscosity of crude oil. But light compounds of crude oil could volatilize at high temperature, and increase the cost of transport. In this paper, the effect of temperature (25 ℃ to 65 ℃) on the fl ow behavior of crude oil was studied at a concentration of 600 mg/L of dendritic long-chain esters. Because the structure of the same generation dendritic long-chain esters was similar, the 0.5 G and 1.5 G dendritic twelve esters were investigated. The results are shown in Figure 3. It was demonstrated that with the increase of temperature, the high molecular weight components of the crude oil, such as asphaltenes, resins, and waxes did not have the chance to agglomerate and form aggregates, while consequently affecting the bonds between the solid particles and eventually reducing the oil viscosity[18]. Judging from the investigation on viscosity versus different temperatures, it was observed that there was a signi fi cant viscosity reduction over the testedtemperatures. This could be attributed to the strong effect of temperature on the viscosity and chemical structure of heavy components of crude oil such as wax (long chain normal alkenes) and asphaltene (hydrogen bonded phenolic OH and nitrogen bases). The viscosity of crude oil with the addition of 1.5G dendritic long-chain esters was lower than that of crude oil with the addition of 0.5 G dendritic long-chain esters. This could occur because the branch chain number of the 1.5 G dendritic long-chain esters was double than that of the 0.5 G dendritic longchain esters, and the long branch chains could effectively adsorb the wax crystals of crude oil and modify the wax crystal structure to prevent them from interlocking and forming three-dimensional networks, thus enhancing the fl ow properties of crude oil.

Figure 3 Effect of temperature on the viscosity of crude oilwith addition of 600 mg/g of dendritic twelve ester


Table 2 Influence of temperature on DVR of the crude oil with addition of dendritic long-chain esters
3.3 In fl uence of dendritic long-chain esters on viscosity of crude oil
The effects of concentration of dendritic long-chain esters on viscosity of crude oil are shown in Figure 4. The presence of dendritic long-chain esters caused strong reduction of apparent viscosity of crude oil. The apparent viscosity reduced with an increasing concentration of dendritic long-chain esters. This could occur because the molecules interacting with the high-molecular compounds in crude oil increased with an increasing concentration ofdendritic long-chain esters, and the reaction could prevent wax and asphaltene from forming hydrogen bonds.
The effects of dendritic long-chain esters on the DVR of crude oil are shown in Figure 5. It can be seen that the DVR increased with an increasing alkyl chain in the dendritic long-chain esters. The reason was probably that the dendritic long-chain ester could form comb-like polymer with the increase of the alkyl chain, and the dispersion of the dendritic long-chain esters in the crude oil could be enhanced. Asphaltenes have hydrogen bonded phenolic OH species and nitrogen bases which can give rise to proton transfer complexes, and the complexes are responsible for the rise in viscosity of oil crude[21]. The polar groups of dendritic long-chain esters, such as ester groups, amide groups and amine groups, may modify the orientation of the aliphatic portion of asphaltene and do not interact with the polar groups that are present in asphaltene complexes to prevent the polar groups of asphaltene from forming hydrogen bonds that would destroy their ordered structures.
Moreover, the polarity of the dendritic long-chain esters would be weakened with the increase of the alkyl chain length, which could make the alkyl chain of the dendritic long-chain ester spread well in the crude oil. The polar groups of the dendritic long-chain esters could spread into the ordered structure of the wax and asphaltene, and might destroy their space network structure.
3.4 In fl uence of dendritic long-chain esters on pour point of crude oil
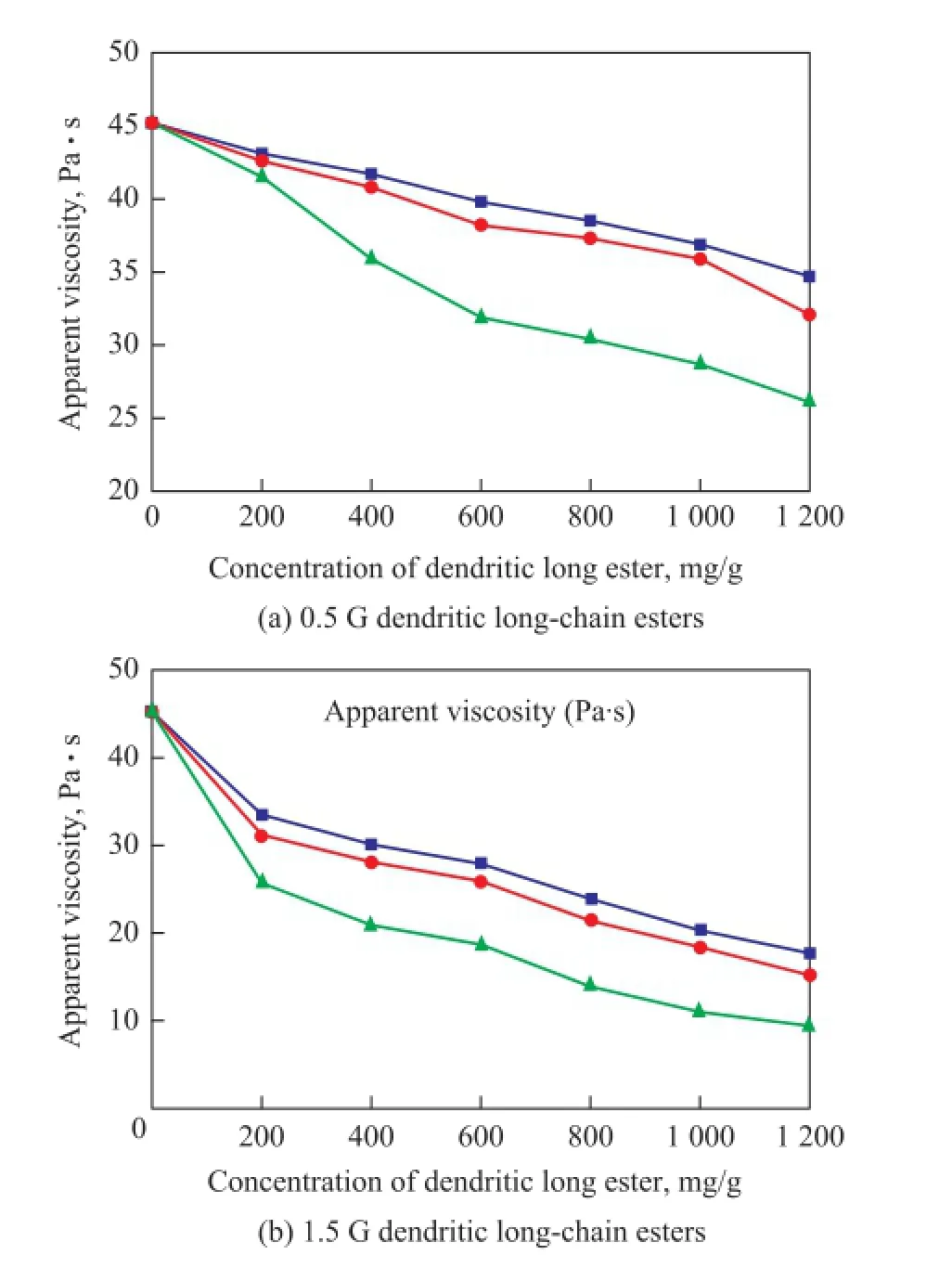
Figure 4 Effect of concentration of dendritic long esters on viscosity of crude oil at 25 ℃ and a shear rate of 60 s-1
All the waxy crude oils eventually become none-fluid on chilling. This was caused by the precipitation of wax crystals in the form of thin plates and needles, which may overlap and interlock with each other to form a threedimensional network. These depressants would obstruct the lateral crystal growth as a result of their adsorption on the precipitating wax[22]. The test results on pour point arepresented in Table 3 as evidenced by pour point reductions in relation to the pour point of the crude oil minus the pour point of crude oil with additives.

Figure 5 Effect of structure of dendritic long-chain ester on DVR of crude oil at a concentration of 600 mg/L
The data in Table 3 indicate that all the dendritic additives were quite effective in reducing the pour point of crude oil. The effectiveness of the dendritic long-chain esters as a pour point depressant depended on the additive concentration, the length of the alkyl side chain and the number of alkyl chains. The effectiveness increased with the increase of the length and the number of the alkyl chains of the dendritic long-chain esters. The reason was that the dendritic long-chain esters functioned by adsorption onto the crystals being formed to redirect their crystal formation and by cocrystallization to form much smaller isotropic crystals and higher solubility wax in the mixed crystals serving as the fl ow improver[23-25]. Under the same conditions, the long alkyl chain could improve the compatibility of the dendritic long-chain esters in the crude oil, and could destroy the formed interlocking network of the waxes.

Table 3 Influence of dendritic long-chain esters on pour point of crude oil
3 Conclusions
Six kinds of dendritic long-chain esters were prepared using ethylamine and alkyl long-chain acrylic esters as starting materials and were investigated as pour point depressants and fl ow improvers for crude oil. The dendritic long-chain esters exhibited satisfactory effects as flow improvers of crude oil. The maximum pour point reduction was obtained when the crude oil was treated by the 1.5 G dendritic eighteen ester, and the minimum pour point reduction was obtained for the crude oil treated by the 0.5 G dendritic eight ester. The longer the chain of dendritic long-chain esters apparently mixed with the paraf fi n chains existing in the crude oil, the better the effect on preventing their facile crystallization. The effects of dendritic long-chain esters on the rheology and flow properties of the crude oil were studied, showing that under the same conditions, the apparent viscosity of the crude oil treated by the 1.5 G dendritic eighteen esters was minimum, while that of the crude oil treated by the 0.5 G dendritic eight esters was maximum. The microcosmic displacement mechanism of the dendritic long-chain esters will be studied in our further work, and the high generation dendritic long-chain esters would be further synthesized and applied to reduce the viscosity of crude oil to enhance the oil fl ow properties.
Acknowledgement: This work was supported fi nancially by the Heilongjiang Postdoctorial Financial Foundation of China (Project NO. LBH-Zo8290). The Daqing Oil Field of China was thanked for providing the fi nancial support and the crude oil.
[1] Hasan S W, Ghannam M T, Esmail N. Heavy crude oil viscosity reduction and rheology for pipeline transportation[J]. Fuel, 2010, 89(5): 1095-1100
[2] Martínez-Palou R, de Lourdes Mosqueira M, Zapata-Rendón B, et al. Transportation of heavy and extra-heavy crude oil by pipeline: A review[J]. Journal of Petroleum Science and Engineering, 2011, 75(3): 274-282
[3] Chang C, Nguyen Q D, R?nningsen H P. Isothermal start-up of pipeline transporting waxy crude oil[J]. Journal of Non-Newtonian Fluid Mechanics, 1999, 87(2): 127-154
[4] Ashrafizadeh S N, Kamran M. Emulsification of heavy crude oil in water for pipeline transportation[J]. Journal of Petroleum Science and Engineering, 2010, 71(3): 205-211
[5] Azodi M, Nazar A R S. An experimental study on factors af-fecting the heavy crude oil in water emulsions viscosity[J]. Journal of Petroleum Science and Engineering, 2013, 106: 1-8
[6] Nassar A M, Ahmed N S. The behavior of α-ole fi ns butyl acrylate copolymers as viscosity index improvers and pour point depressants for lube oil[J]. International Journal of Polymeric Materials, 2006, 55(11): 947-955
[7] Deshmukh S, Bharambe D P. Synthesis of polymeric pour point depressants for Nada crude oil (Gujarat, India) and its impact on oil rheology[J]. Fuel Processing Technology, 2008, 89(3): 227-233
[8] Farag R K. Poly (cinnamoyloxy ethyl methacrylate-co-octadecyl acrylate) as fl ow improver for Egyptian waxy crude oils[J]. International Journal of Polymeric Materials, 2008, 57(3): 189-202
[9] Castro L V, Vazquez F. Copolymers as fl ow improvers for Mexican crude oils[J]. Energy & Fuels, 2008, 22(6): 4006-4011
[10] Ashbaugh H S, Guo X, Schwahn D, et al. Interaction of paraffin wax gels with ethylene/vinyl acetate copolymers[J]. Energy & fuels, 2005, 19(1): 138-144
[11] Khidr T T. Synthesis and evaluation of copolymers as pour-point depressants[J]. Petroleum Science and Technology, 2007, 25(5): 671-681
[12] Lee C C, MacKay J A, Fréchet J M J, et al. Designing dendrimers for biological applications[J]. Nature Biotechnology, 2005, 23(12): 1517-1526
[13] Kurtoglu Y E, Navath R S, Wang B, et al. Poly (amidoamine) dendrimer–drug conjugates with disul fi de linkages for intracellular drug delivery[J]. Biomaterials, 2009, 30(11): 2112-2121
[14] Shi Zehua, Shu Xin, Chen Dongzhong. Rationally designed syntheses and self-organized superstructure investigations of liquid crystalline dendrimers[J]. Progress in Chemistry, 2009, 21(7/8): 1534-1545 (in Chinese)
[15] Chattopadhyay S, Chakraborty P, Drew M G B, et al. Nickel (II) complexes of terdentate or symmetrical tetradentate Schiff bases: Evidence of the in fl uence of the counter anions in the hydrolysis of the imine bond in Schiff base complexes[J]. Inorganica Chimica Acta, 2009, 362(2): 502-508
[16] Giraldo S Y, Rios L A, Suárez N. Comparison of glycerol ketals, glycerol acetates and branched alcohol-derived fatty esters as cold- fl ow improvers for palm biodiesel[J]. Fuel, 2013, 108: 709-714
[17] Wang J, Li C Q, Qu H J, et al. Terminal group effects on demulsification using dendrimers[J]. Petroleum Science and Technology, 2010, 28(9): 883-891
[18] Machado A L C, Lucas E F, González G. Poly-(ethyleneco-vinyl acetate) (EVA) as wax inhibitor of a Brazilian crude oil: oil viscosity, pour point and phase behavior of organic solutions[J]. Journal of Petroleum Science and Engineering, 2001, 32(2): 159-165
[19] Ghannam M T, Hasan S W, Abu-Jdayil B, et al. Rheological properties of heavy & light crude oil mixtures for improving fl owability[J]. Journal of Petroleum Science and Engineering, 2012, 81: 122-128
[20] Ghannam M T, Esmail N. Flow enhancement of mediumviscosity crude oil[J]. Journal of Petroleum Science and Technology, 2006, 24(8): 985-999
[21] Ha fi z A A, Khidr T T, Hexa-triethanolamine oleate esters as pour poit depressant for waxy crude oil[J]. Journal of Petroleum Science and Technology, 2007,56(4):296-302
[22] Khan M R. Rheological properties of heavy oils and heavy oil emulsions[J]. Energy Sources, 1996, 18(4): 385-391
[23] Deshmukh S, Bharambe D P. Synthesis of polymeric pour point depressants for Nada crude oil (Gujarat, India) and its impact on oil rheology[J]. Fuel Processing Technology, 2008, 89(3): 227-233
[24] Li Qianshu, He Jianxun.Research on synthesis of pour point depressant polymethacrylate for lubricating oil[J]. Petroleum Processing and Petrochemicals, 2015, 46(6): 84-88 (in Chinese)
[25] Song Linhua, Ni Bin, Wu Xinpeng. Synthesis of tetramer MSAZ and application in viscosity reduction for heavy crude oil with surfactant[J]. Petroleum Processing and Petrochemicals, 2015, 46(6): 79-83 (in Chinese)
Received date: 2015-09-18; Accepted date: 2015-11-22.
Dr. Li Cuiqin, Telephone: +86- 459-6504224; E-mail: dqpilicuiqin@126.com.
- 中國煉油與石油化工的其它文章
- Experimental Study of UDS Solvents for Purifying Highly Sour Natural Gas at Industrial Side-stream Plant
- Highly Active and Stable Ni2P/SiO2Catalyst for Hydrogenation of C9Petroleum Resin
- Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
- Investigation of Different Coke Samples Adhering to Cyclone Walls of a Commercial RFCC Reactor
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Preparation of Core-Shell Composite of Y@Mesoporous Alumina and Its Application in Heavy Oil Cracking

