Study on Absorption and Regeneration Performance of Novel Hybrid Solutions for CO2Capture
Gao Jie; Yin Jun; Zhu Feifei; Chen Xin; Tong Ming; Kang Wanzhong; Zhou Yanbo; Lu Jun
(1. Key Laboratory of Coal Gasi fi cation and Energy Chemical Engineering of Ministry of Education, East China University of Science & Technology, Shanghai 200237; 2. SINOPEC Ningbo Engineering Co., Ltd., Ningbo 315103)
Study on Absorption and Regeneration Performance of Novel Hybrid Solutions for CO2Capture
Gao Jie1; Yin Jun1; Zhu Feifei1; Chen Xin2; Tong Ming2; Kang Wanzhong2; Zhou Yanbo1; Lu Jun1
(1. Key Laboratory of Coal Gasi fi cation and Energy Chemical Engineering of Ministry of Education, East China University of Science & Technology, Shanghai 200237; 2. SINOPEC Ningbo Engineering Co., Ltd., Ningbo 315103)
Recently, a kind of hybrid solution MEA-methanol shows a better CO2capture performance over aqueous MEA solution. However, the vaporization of methanol is the biggest disadvantage that hinders its application, so it is necessary to minimize the vaporization of methanol during both the absorption and regeneration processes. In this work, two kinds of hybrid solutions were studied and compared with aqueous MEA solution and MEA-methanol solution, including MEA/TEA/methanol solution and MEA/glycerol/methanol solution. The absorption property of MEA/glycerol/methanol solution is better than aqueous MEA solution within a certain period of time and the absorption property of MEA/TEA/methanol solution is too poor to be used in CO2capture. By increasing the concentration of TEA and decreasing the concentration of MEA, the absorption rate, CO2capture ef fi ciency and absorption capacity all decreased. Upon adding glycerol, the cyclic capacity decreased and the generation temperature increased, and moreover, the density and viscosity also increased considerably. So after adding TEA and glycerol, the CO2capture performance of MEA-methanol solvent cannot be improved.
CO2capture; MEA; methanol; glycerol; hybrid solvent
1 Introduction
Carbon capture and storage (CCS), speci fi cally the postcombustion capture (PCC) technologies, have been identi fi ed as a crucial and key strategy in the development and implementation of short to intermediate term carbon reduction strategies from coal-fired power generation[1]. A number of capture techniques have been demonstrated to successfully capture CO2using chemical absorption process. Absorption solvents can react with CO2to form carbamates, carbonates or hydrogen carbonates[2]. Once captured, the CO2is typically recovered by a reversal of the chemical reaction between amine and CO2, which is induced by a temperature increase, a reduction in pressure or both[3]. The use of aqueous solutions of amines is the most widespread technology in the gas treating process[4]. And a variety of solvents have been used in the CO2absorption process[5]. However, the conventional alkanolamines such as monoethanolamine (MEA), diethanolamine (DEA),N-methyldiethanolamine (MDEA), and 2-amino-2-methyl-1-propanol (AMP) require very high regeneration energy cost. The regeneration energy of the aforementioned solvents is about 3.2—4.0 GJ/ton of CO2, which would decrease the generating efficiency of the fossil fuel-fired power plants[6]. Moreover, the aqueous MEA solution, which is widely used as a standard to evaluate the CO2absorption performance, has a low capacity and a high energy cost despite major improvements that have been made on the process side like lean vapor compression and intercooling[7-8]. So it is essential to find new efficient solvents, which are considered to have fast reaction kinetics, high absorption capacity, low regeneration energy, high stability and low corrosiveness to secure the economic feasibility of chemical absorption process[9].
Numerous solvent systems aiming to address the underlying shortcomings with the absorption behavior and chemical robustness experienced with existing solvent systems have been proposed[10]. Blended amines such as MEA-MDEA, MEA-AMP, MEA-TEA, MEAPZ, AMP-PZ and MEA-MDEA[11-13], the newly developed amines such as 1-diethylamino-2-propanol (1DEA2P), 4-diethylamino-2-butanol (DEAB), diethylenetriamine (DETA), and inorganic solvents such as aqueous ammonia (NH3) and potassium carbonate (K2CO3) have been studied by many researchers[14-16]. One of the main benefits of these new solvents is that they combine the advantages of the fast kinetics of the primary amines and the high absorption capacity and low energy consumption for solvent regeneration of the tertiary amines, sterically hindered amines or inorganic solvents. However, the regeneration process of these solvents are carried out at elevated temperatures, typically over 373 K[16].
Recently, hybrid solutions which are formed by blending chemical solvents and physical solvents have been proposed for CO2absorption such as aniline in ethanol, and 3-amino-1-propanol in methanol and ethanol. These studies showed that hybrid solutions have significant advantages over corresponding chemical solvents. Methanol is the most widely used physical solvent for CO2capture from natural gas and synthesis gas due to its high physical solubility of CO2and low heat consumption in the regeneration process and the hybrid of MEA and methanol has showed a better performance over aqueous MEA solutions. However, vaporization of methanol is the biggest disadvantage that hinders its application due to the high vapor pressure of methanol, so it is necessary to minimize the vaporization in the absorption and regeneration processes[17-18].
In this work, triethanolamine (TEA) and glycerol were added into the MEA-methanol solution, respectively, to study their performance in minimizing the vaporization of methanol. Also the absorption performance, regeneration performance and physical performance of new hybrid solutions were studied in comparison with aqueous MEA solution and MEA-methanol solution to evaluate their performance for CO2capture.
2 Experimental
2.1 Materials
CO2and N2gases with mole fractions of 0.999 and 0.999 were supplied by the Shanghai Shenkai Gas Company. Analytically pure grade MEA (with a purity>99.9%), TEA (with a purity>78%), glycerol (with a purity >98%) and methanol (with a purity >99%) were all used as purchased without further purification. The aqueous solution was prepared using distilled water.
2.2 CO2absorption experiments
A semi-batch absorption apparatus which was used to measure the absorption performance of different solutions is shown in Figure 1. This experimental apparatus consisted mainly of three parts: gas supply, absorption reactor and gas detection. Detailed operating conditions of different solvents are summarized in Table 1.
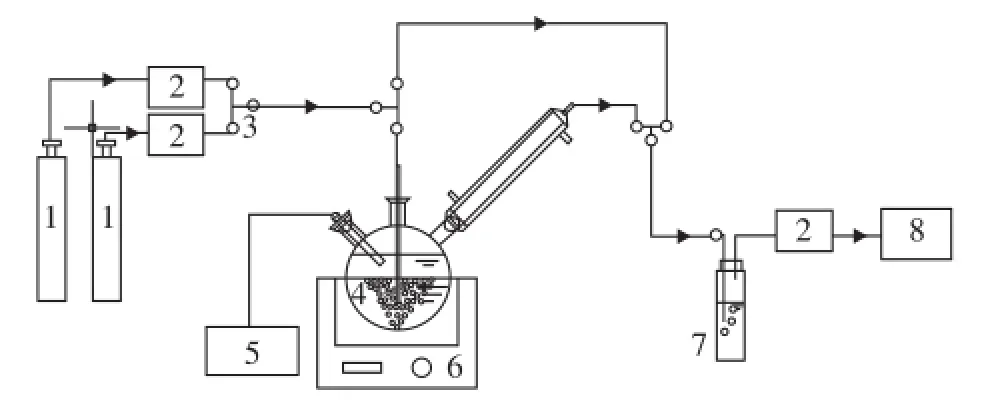
Figure 1 Experimental apparatus for CO2absorption

Table 1 Absorption operating parameters
Firstly, the solution with a volume of 500 mL was put into the reactor at the desired temperature. Then the gas fl ow rates of N2and CO2gases were controlled by mass fl ow controllers before being remixed, while the total flow rate was controlled at 0.8 L/min. The flue gas was thenrouted through the by-pass tube and tested by the fl ue gas analyzer to maintain the CO2concentration at 15%. As the desired temperature of the reactor and concentration of CO2were achieved, the valve was opened to inject the flue gas into the reactor to start the absorption experiment. The fl ow rate of in fl uent gas and ef fl uent gas from the reactor, the pH value of the solution and the CO2concentration released from the reactor were measured and recorded. When the solution was saturated, the absorption process would be stopped. The CO2absorption rate was then determined from the difference between the flow rates of the influent and effluent gas based on the following formula:
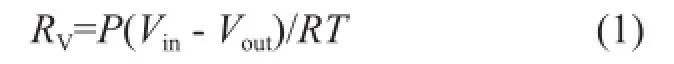
whereRVis the CO2mole absorption rate, the unit of which is mol/s, P is the atmospheric pressure,VinandVoutrepresent the volume fl ow rate of inlet gas and outlet gas, respectively, andTis the reaction temperature.

whereYinandYoutare CO2concentration of inlet and outlet gas, respectively[19].
2.3 CO2regeneration experiments
In the course of thermal regeneration, the temperature of the oil bath was heated from room temperature to 140 ℃, which was then maintained at this level for all the solutions. A three-necked fl ask placed in the oil bath was fed with 500 mL of saturated solution. Two condensers were connected to the flask to minimize the evaporation loss of the solution. The temperature of the solution during the regeneration process was measured through a thermometer. The released gas was led to a bottle full of water to knock out the aqueous vapor before the outlet fl ow rate was measured. The temperature of the solution and the outlet gas, as well as the gas flow rate were measured and recorded every minute simultaneously.
2.4 Density and viscosity
Density and viscosity are very important factors in evaluating the performance of different solutions for CO2capture. The density of solutions was measured using a pycnometer. Before the density measurements, volume of the pycnometer was measured with distilled water. In each run, the pycnometer containing the solution was put in a water bath at the desired temperature. The density data are the average of three measurements.
The viscosity of different solutions was measured with a Ukrainian-style viscometer. For each measurement, the viscometer was placed in a water bath to maintain the desired temperature. A sample was put into the viscometer and was allowed to fl ow through the capillary tube, while the time was measured by a stopwatch. Each viscosity data are the average of three measurements.
3 Results and Discussions
3.1 Absorption performance of novel hybrid solutions
In order to evaluate the absorption performance of novel hybrid solutions (including 20% MEA/10 % TEA/ 70% methanol, 10% MEA/20 % TEA/ 70% methanol, and 30% MEA/30% glycerol/ 40% methanol), the 30 % MEA/70% H2O solution and the 30% MEA/70% methanol solution were also studied in a semi-batch absorption apparatus separately. Figure 2 shows the absorption rate (a), CO2capture efficiency (b), absorption capacity (c) and pH value (d) curves of different solutions. It can be observed that the initial absorption rate of all the hybrid solutions is higher than that of 30 % MEA aqueous solution which shows the advantage of hybrid solutions. As the reaction time increases, the absorption rates of all solutions decrease, while after about 125 min the absorption rate of 10% MEA/20% TEA/70% methanol decreases to 1×10-5mol/s; and after about 175 min the absorption rate of 20% MEA/10% TEA/70% methanol decreases to 1×10-5mol/s. However, a reaction duration of 230 min, 250 min and 280 min is needed for reducing the absorption rate of 30% MEA/70% methanol, 30% MEA/30% glycerol/40% methanol, and 30% MEA/70% H2O, respectively, to 1×10-5mol/s. So it can be known that with the increase of TEA concentration and the decrease of MEA concentration, the absorption rate decreases more quickly because the tertiary amine is unable to react with CO2in the absence of water[18]. And upon adding glycerol, the reaction time of the solution increases, but the absorption rate decreases compared to that of the MEA/methanol solution. Figure 2(b) shows the same tendency with absorption rate curve and it canbe seen that within 160 min, the capture efficiency of 30% MEA/30% glycerol/40% methanol is above 80%, which is perfect for CO2capture. Figure 2(c) shows that the absorption capacity of 30% MEA/30% glycerol/40% methanol is almost the same with 30% MEA/70% methanol, and the absorption capacity of MEA/TEA/methanol solvent is too low to be used in CO2capture. Also it can be seen from Figure 2(d) that a high pH value represents a high absorption rate.
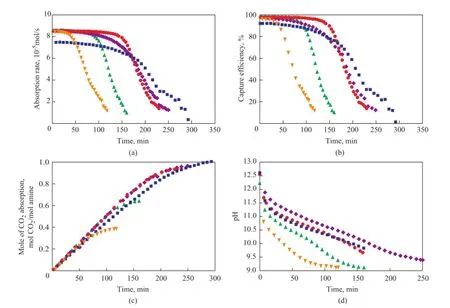
Figure 2 Changes in CO2absorption rate (a), CO2capture ef fi ciency (b), CO2absorption capacity (c), and pH value (d) of different solutions with reaction time
Therefore, the absorption property of MEA/glycerol/methanol solution is better than aqueous MEA solution in a certain period of time and the absorption property of MEA/TEA/methanol solution is too poor to be used in CO2capture.
3.2 Regeneration performance of novel hybrid solutions
The regeneration temperature is directly associated with the energy required to regenerate solvents and is an important factor for selecting solvents[20]. Figure 3 shows the regeneration rates of different solvents changing with the different regeneration temperature. It can be observed that the regeneration rates increase with an increasing temperature, and the maximum regeneration rates of MEA/methanol,MEA/TEA/methanol, MEA/glycerol/methanol and MEA/H2O solutions are achieved at 60 ℃, 65 ℃, 70 ℃, and 100 ℃, respectively. Although the regeneration temperature can be increased by adding glycerol, still the regeneration temperature of MEA/glycerol/methanol is much lower than that of the aqueous MEA solution.
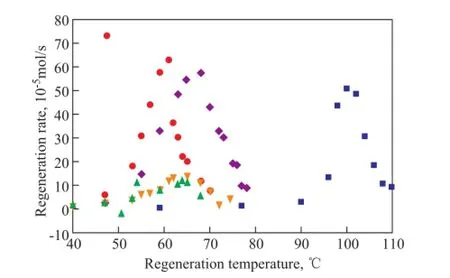
Figure 3 CO2regeneration rates of different solvents changing with regeneration temperatures
3.3 Cyclic absorption performance of novel hybrid solutions
Because of the needs for repeated use of solvents in the industry, the cyclic absorption capacity becomes a very important factor in evaluating a solvent for the industrial use[21]. The CO2absorption capacity of the fresh solvents (first cycle), the solvents after being subject to regeneration once (second cycle), and solvents after being subject to regeneration twice (third cycle) was studied in this work, when these solvents were used in the absorption operation over the same reaction duration. The results are shown in Figure 4. It can be observed that after the second cycle, the absorption capacity of 20% MEA/10% TEA/70% methanol, 10% MEA/20% TEA/70% methanol, 30% MEA/30% glycerol/40% methanol, 30% MEA/70% H2O, and 30% MEA/70% methanol decreases by 0.278 7 mol CO2/mol amine, 0.045 4 mol CO2/mol amine, 0.429 5 mol CO2/mol amine, 0.358 5 mol CO2/mol amine, and 0.297 1 mol CO2/mol amine, respectively, as compared to that of the fresh solvents in the fi rst cycle. And after the third cycle, the absorption capacity decreases by 0.057 1 mol CO2/mol amine, 0.043 0 mol CO2/mol amine, 0.118 3 mol CO2/mol amine, 0.018 3 mol CO2/mol amine, and 0.020 0 mol CO2/mol amine, respectively, compared to that of solutions after the second cycle. Based on these data, we can know that TEA has a better cyclic absorption property than MEA and the cyclic absorption capacity decreases after adding glycerol.
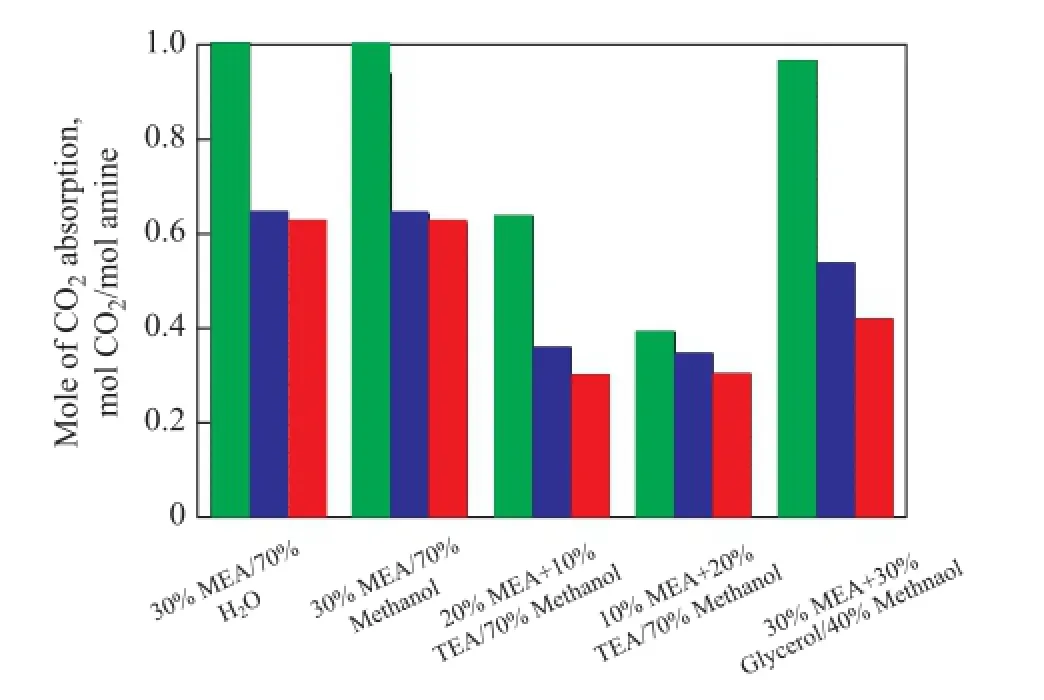
Figure 4 CO2cyclic absorption capacities of different solvents
3.4 Density and viscosity
The overall assessment of a solvent for CO2capture requires the knowledge of its physical properties. And the density and viscosity data are very important physical properties which are associated with the pumping energy cost of the circulating solvent in the absorptionregeneration system[22].
The density and viscosity of lean and rich solutions composed of 30% MEA/70% H2O operating at 40 ℃, and 20% MEA/10% TEA/70% methanol, 10% MEA/20% TEA/70% methanol, 30% MEA/30% glycerol/40% methanol, and 30% MEA/70% methanol operating at 298 K were measured. The detailed results are shown in Table 2 and Figure 5 in order to compare these data more clearly. Based on these data, we can know that the rich solution of MEA/TEA/methanol has a relatively low density and viscosity resulting in its low CO2absorption capacity, while the rich MEA/glycerol/methanol solution has the highest density and viscosity which will consume a lot of energy.
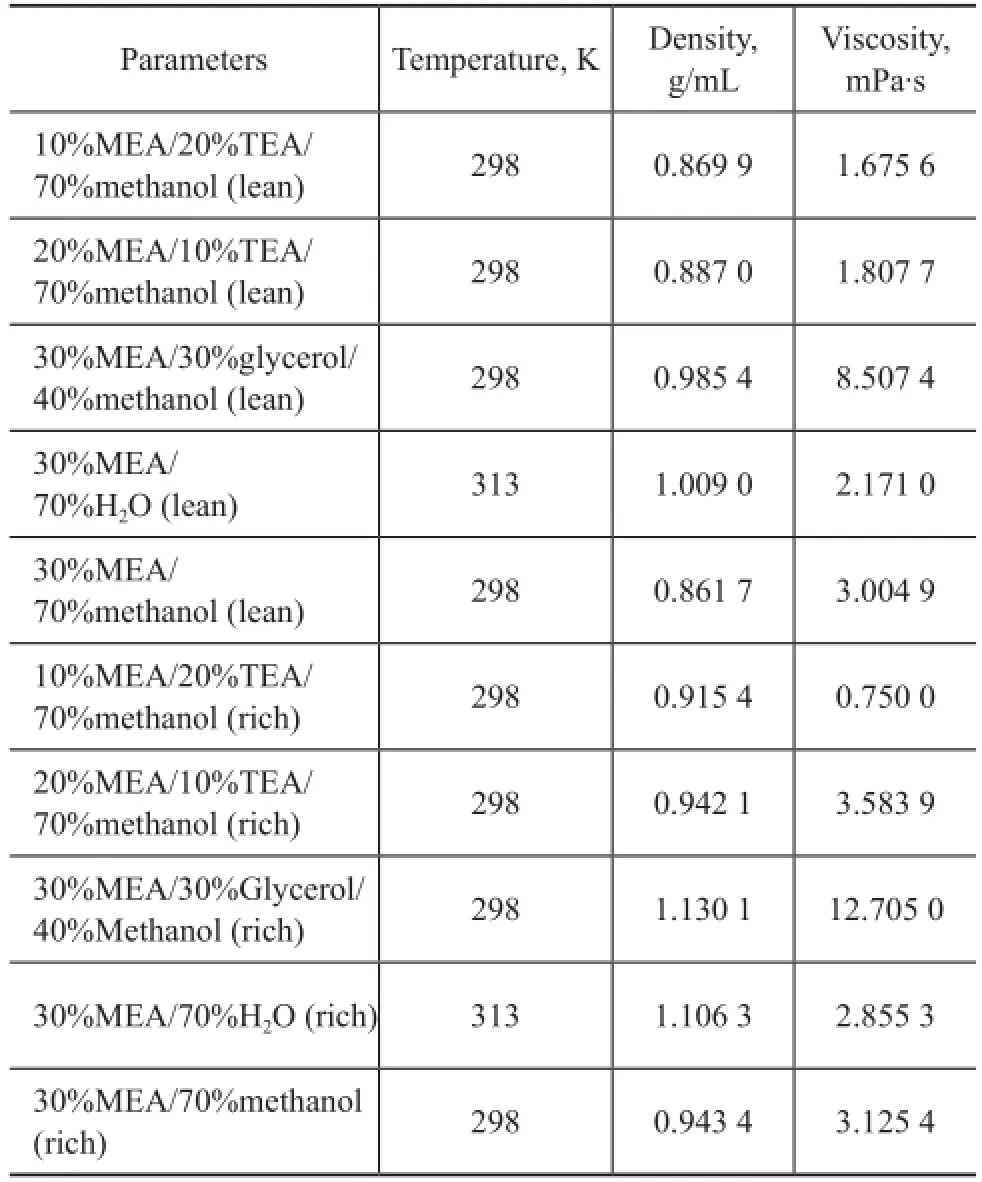
Table 2 Density and viscosity of different solvents
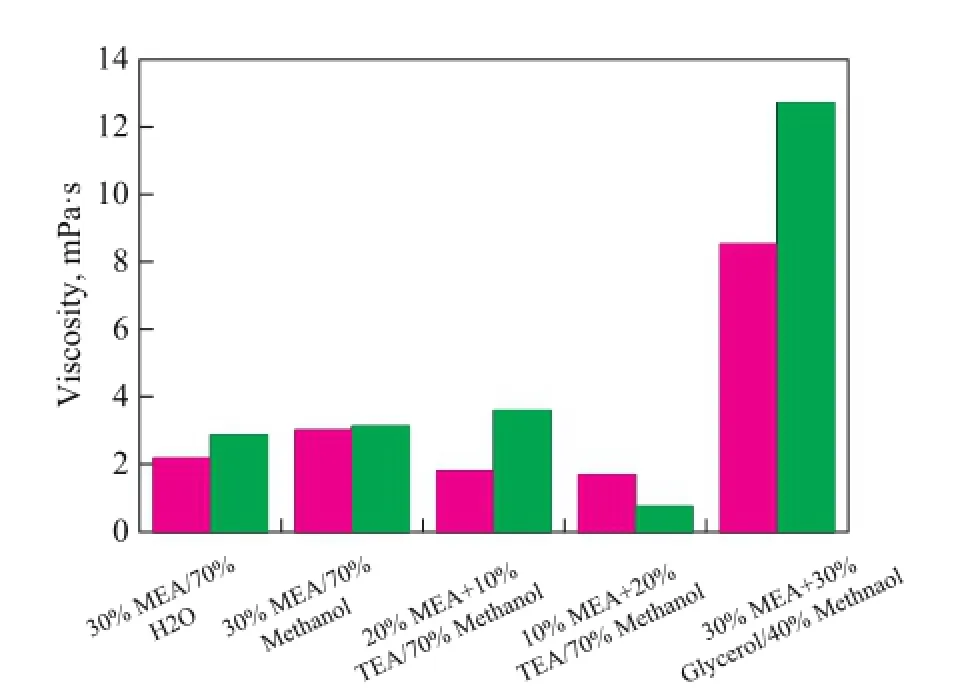
Figure 5 Density and Viscosity of different solvents
4 Conclusions
The CO2absorption performance, regeneration performance, cyclic absorption performance and physical properties of novel hybrid solutions, including 20% MEA/10% TEA/70% methanol, 10% MEA/20% TEA/70% methanol and 30% MEA/30% glycerol/40% methanol were studied compared with 30 % MEA/70% H2O and 30 % MEA/70% methanol solvents. It was found that by increasing the concentration of TEA and decreasing the concentration of MEA, the absorption rate, CO2capture efficiency and absorption capacity all decreased. Upon adding glycerol, the cyclic capacity decreased and regeneration temperature increased, and moreover, the density and viscosity increased considerably. Judging from the overall experiments, it can be concluded that tertiary amines should not be used in non-aqueous solution and addition of glycerol cannot improve the performance of MEA-methanol solution and more studies are needed to minimize the methanol vaporization in the absorption and regeneration processes.
Acknowledgement: This work was supported by the Sinopec Ningbo Engineering Co., Ltd. (No. 14850000-14-ZC0609-0003, H8XY-0032).
[1] Leung D Y C, Caramanna G, Maroto-Valer M M. An overview of current status of carbon dioxide capture and storage technologies [J]. Renewable and Sustainable Energy Reviews, 2014, 39: 426-443
[2] Wang M, Lawal A, Stephenson P. Post-combustion CO2capture with chemical absorption: a state-of-the-art review [J]. Chemical Engineering Research and Design, 2011, 89(9): 1609-1624
[3] Ludovic D, Colin S, Geoff S. Puri fi cation of aqueous amine solvents used in post combustion CO2capture: A review [J]. International Journal of Greenhouse Gas Control, 2012, 10: 443-455
[4] Nathalie J M C, Penders-van E, Sylvie F. Effect of pKa on the kinetics of carbon dioxide absorption in aqueous alkanolamine solutions containing carbonic anhydrase at 298 K [J]. Chemical Engineering Journal, 2015, 259(1): 682-691
[5] Marcin S, Adam T, Lucyna W. Pilot plant results for advanced CO2capture process using amine scrubbing at the Jaworzno power plant in Poland [J]. Fuel, 2015, 151(1): 50-56
[6] Jeon S B, Cho S W, Lee S W. Absorption characteristics of carbon dioxide into an O/W emulsion absorbent containing N-methylcyclohexylamine/2,6-dimethylpiperidine [J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(5): 2673-2680
[7] Plaza J M, Chen E, Rochelle G T. Absorber intercooling in CO2absorption by piperazine-promoted potassium carbonate [J]. AIChE Journal, 2010, 56: 905-914
[8] Hari P M, Hans, H. Pilot plant study of two new solvents for post combustion carbon dioxide capture by reactive absorption and comparison to monoethanolamine [J]. Chemical Engineering Science, 2011, 66(22): 5512-5522
[9] Kazuya G, Hiromichi O, Firoz A C. Development of novel solvents for CO2capture from blast furnace gas [J]. International Journal of Greenhouse Gas Control, 2011, 5(5): 1214-1219
[10] William C, Yaser B, Gilles R. Rapid CO2absorption into aqueous benzylamine (BZA) solutions and its formulations with monoethanolamine (MEA), and 2-amino-2-methyl-1-propanol (AMP) as components for post combustion capture processes [J]. Chemical Engineering Journal, 2015, 264(15): 954-961
[11] Puxty G, Rowland R. Modeling CO2mass transfer in amine mixtures: PZ-AMP and PZ-MDEA [J]. Environmental Science and Technology, 2011, 45: 2398-2405
[12] Sema T, Naami A, Fu K. Comprehensive mass transfer and reaction kinetics studies of CO2absorption into aqueous solutions of blended MDEA-MEA [J]. ChemicalEngineering Journal, 2012, 209(15): 501-512
[13] Sema T, Naami A, Fu K. Comprehensive mass transfer and reaction kinetics studies of a novel reactive 4-diethylamino-2-butanol solvent for capturing CO2[J]. Chemical Engineering Science, 2013, 100(30): 183-194
[14] Zhang X, Fu K, Liang Z. Experimental studies of regeneration heat duty for CO2desorption from diethylenetriamine (DETA) solution in a stripper column packed with Dixon ring random packing [J]. Fuel, 2014, 136(15): 261-267
[15] Kim G H, Park S Y, You J K. CO2absorption kinetics in a CO2free and partially loaded aqueous ammonia solution [J]. Chemical Engineering Journal, 2014, 250(15): 83-90
[16] Zhao B T, Su Y X, Tao W W. Post-combustion CO2capture by aqueous ammonia: a state-of-the-art review [J]. International Journal of Greenhouse Gas Control, 2012, 9: 355-371
[17] Fu K Y, Wong W R, Liang Z W. Experimental analyses of mass transfer and heat transfer of post-combustion CO2absorption using hybrid solvent MEA-MeOH in an absorber [J]. Chemical Engineering Journal, 2015, 260(15): 11-19
[18] Barzagli F, Lai S, Mani F. Novel non-aqueous amine solvents for reversible CO2capture [J]. Energy Procedia, 2014, 63: 1795-1804
[19] Chen H Y, Chuang S T. Mixed alkanolamines with low regeneration energy for CO2capture in a rotating packed bed [J]. Energy Procedia, 2013, 37: 455-460
[20] Kim Y E, Moon S J, Yoon I Y. Heat of absorption and absorption capacity of CO2in aqueous solutions of amine containing multiple amino groups [J]. Separation and Puri fi cation Technology, 2014, 122(10): 112-118
[21] Zhang J F, Misch R, Tan Y. Novel thermomorphic biphasic amine solvents for CO2absorption and low-temperature extractive regeneration [J]. Chemical and Engineering Technology, 2011, 9: 1481-1489
[22] Muraleedlharan R, Mondal A, Mandal B. Absorption of carbon dioxide into aqueous blends of 2-amino-2-hydroxymethyl-1,3-propanediol and monoethanolamine [J]. Separation and Puri fi cation Technology, 2012, 94(19): 92-96
Received date: 2015-10-14; Accepted date: 2015-11-25.
Prof. Lu Jun, Telephone: +86-021-64252443, Fax: +86-021-64252737; E-mail: lujun@ecust.edu.cn.
- 中國煉油與石油化工的其它文章
- Experimental Study of UDS Solvents for Purifying Highly Sour Natural Gas at Industrial Side-stream Plant
- Highly Active and Stable Ni2P/SiO2Catalyst for Hydrogenation of C9Petroleum Resin
- Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
- Investigation of Different Coke Samples Adhering to Cyclone Walls of a Commercial RFCC Reactor
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Preparation of Core-Shell Composite of Y@Mesoporous Alumina and Its Application in Heavy Oil Cracking

