Effects of Silylation on Zn-IM5 and Its Catalytic Activity for Butane Aromatization
Yu Lei; Yi Dezhi; Lu Yannan; Shi Li; Chen Junwen; Meng Xuan
(1. The State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237; 2. SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Effects of Silylation on Zn-IM5 and Its Catalytic Activity for Butane Aromatization
Yu Lei1; Yi Dezhi1; Lu Yannan1; Shi Li1; Chen Junwen2; Meng Xuan1
(1. The State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237; 2. SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Effects of silylation on surface properties and catalytic performance of Zn-IM5 for butane aromatization were studied in this paper. Collidine-IR and NH3-TPD analyses revealed that the silylation treatment not only decreased the quantity of both strong and weak acid sites but also led to a slightly reduced intensity of weak acidity. Silylation of the catalyst promoted the selectivity of BTX by narrowing the channel and cutting the acidity. The effect of temperature of silylation and amount of Si loading were evaluated. The best condition has speci fi ed a temperature of 50 ℃ and a SiO2loading of 4.0%.
silylation; Zn-IM5; aromatization.
1 Introduction
Aromatics have been widely used in the production of fuel, fiber and drugs. Currently, aromatic hydrocarbons are mainly obtained from catalytic reforming process which needs a certain content of cycloalkanes. This limits the production of aromatics from the source. On the other hand, the growing scarcity of oil resources urges researchers to provide new routes for the production of aromatic hydrocarbons. Many efforts have been made to produce aromatics by dehydrogenation and aromatization of light alkanes[1-3]. Several aromatization processes were developed for converting light alkanes to aromatics, such as the M2 Forming developed by Mobil and the Cyclar process developed by UOP and BP[4]. Large amounts of C4hydrocarbons are produced as by-products in catalytic cracking units, which contain a massive amount of butane. The transformation of butane to aromatics is always attractive for a long time. Many attempts were made to improve the conversion and light aromatics selectivity, such as introduction of a second metal component[5-8]and external surface modi fi cation. Recently, Tempelman C H L, et al.[9]reported an increased conversion and benzene selectivity in methane aromatization over Si-modified catalysts. It was con fi rmed that the hard coke layer formed on the external surface can be partially deactivated by silylation[10]. It seems that the introduction of Si species is a feasible way for promoting the catalytic activity of aromatization.
A high-silica zeolite with an unusual channel system had been reported in 1998 by Benazzi, et al.[11], and the detailed crystal structure was not fully analyzed until 2007[12]. There have been many researchers fl inging themselves into the exploration of IM-5[13-14]. And exploration of the potential capacity of IM-5 in the field of butane aromatization is one of the focuses of this paper.
Pore structure and acid sites were identified as the key factors for aromatization[15-16]. And a proper modi fi cation for these two elements can be helpful to the aromatization reaction, since the intrinsic composition cannot meet the requirements very well. It is our intention to provide data to prove that the modification of the pore structure and catalytic acidity by silylation can be bene fi cial to the aromatization reaction.
2 Experimental
2.1 Catalyst synthesis
Na-form IM5 zeolite powder with a SiO2/Al2O3ratio of 48 (provided by the SINOPEC Research Institute of Petroleum Processing, China) was calcined in air at 550 ℃ for 5 h to remove the template. Then the zeolite was exchanged with a 1 mol/L ammonium nitrate solution at 80 ℃ for 10 h, fol-lowed by washing, drying and calcination to produce the H-IM5 zeolite. For achieving Zn loading, the zeolite was impregnated (via the incipient wetness impregnation process) with an aqueous solution of Zn(NO3)2(corresponding to 2.2% of Zn on the zeolite). The resulting materials were dried at 120 ℃ overnight, and then calcined at 550 ℃ for 3 h. The silylation of Zn-IM5 zeolite was conducted following the method reported by Zheng, et al.[17]Typically, 2 g of Zn-IM5 zeolite was dried at 120 ℃ overnight prior to adding 50 mL ofn-hexane. To this suspension, 0.3 mL of TEOS (corresponding to 4.0 wt% of SiO2on the zeolite) was introduced and the silylation was carried out at 80 ℃ for 1.0 h under stirring and re fl ux. After that,n-hexane was discharged by removing the reflux and the catalyst was dried overnight at 120 ℃, prior to being subject to calcination at 550 ℃ for 3 h. Finally, samples obtained thereby were extruded with γ-alumina (20%) to make the slurry, followed by drying overnight and calcination at 600℃ for 3 h.
2.2 Catalytic reaction
The reaction of butane aromatization was evaluated in a continuous fl ow fi xed-bed reactor (with an i. d. of 5 mm) at 570 ℃ and 0.65 MPa with a WHSV of 0.3 h-1. Prior to the reaction, the catalyst was pretreated under a dry air fl ow at a rate of 30 mL/min at 550 ℃ for 1 h. Butane was fed into the reactor when the catalyst bed was preheated to the required reaction temperature. The product after being cooled down in a condensate tank entered a gas–liquid separator, and was periodically discharged. Liquid fractions were analyzed by a GC (Agilent GC6890-MS 5973 N) equipped with a FFAP capillary column and the gas was analyzed by GC7900 equipped with a S/Al2O3plot capillary column.
The yield in the paper was equal to the product of butane conversion and BTX selectivity.
2.3 Catalyst characterization
2.3.1 SEM
Scanning electron micrographs were obtained on a Nova NanoSEM 450 scanning electron microscope operating at an electron acceleration voltage of 20.0 kV.
2.3.2 X-ray diffraction (XRD)
XRD patterns were obtained on a Simens D-500 diffractometer using Cu Kα radiation at a tube voltage of 40 kV and a tube current of 40 mA with a scanning speed of 5(°)/min.
2.3.3 Thermal gravimetric analysis (TG)
The coke content of spent catalyst sample after 12 h on stream was measured by a SDT Q600 TG/DTA thermal analyzer when it was preheated at a heating rate of 10 ℃/min from room temperature to 800 ℃ in an air fl ow.
2.3.4 NH3-temperature programmed desorption (NH3-TPD)
The NH3-TPD was used to determine the acidity of the sample. Prior to the measurements, 0.1 g of sample were pretreated at 600 ℃ for 1 h in a fl owing He gas. Then, the sample was cooled down to 50 ℃ and was blanketed with NH3gas. When the baseline was stable, the NH3-TPD began to run under a constant fl ow of He stream from 50 ℃to 700 ℃ at a heating rate of 10 ℃/min. The concentration of NH3in the exit gas was monitored continuously by a gas chromatograph out fi tted with a TCD.
2.3.5 BET analysis
Nitrogen adsorption isotherms were obtained on a Micromeritics JW-BK200C surface area and pore size analyzer at -196 ℃ in the static measurement mode. The sample was outgassed at 350 ℃ for 5 h prior to the measurements. Speci fi c surface area was calculated with the help of BET equation. The information about micropores was evaluated by means of the t-plot method, while the BJH method could be used to investigate the mesopores.
2.3.6 Collidine-IR
The amount of acid sites on the external surface of the zeolite was obtained via the Collidine-IR spectroscopy (Magna-IR550, Nicolet Company), using 2,4,6-collidine (Coll, 7.4 ? in diameter) as the probe molecule. Firstly, the sample powder was pressed into self-supporting wafer and placed into a quartz IR cell outfitted with KBr windows. The adsorption proceeded at room temperature for 1.5 h after the wafer was dehydrated under vacuum at 380 ℃ for 2 h, and then the desorption was carried out at 200 ℃ and 450 ℃ for 2 h, respectively. The total amount of acid sites was represented by the adsorption of 2,4,6-collidine after desorption at 200 ℃; and the amountof strong acid sites was measured after desorption at 450 ℃. According to previous literature[18], bands at 1 632—1 648 cm-1were attributed to the acid sites on the external surface.
3 Results and Discussion
3.1 SEM
The SEM image in Figure 1 shows that the particles assumed a rod-like shape with a typical size of 400 nm×50 nm.

Figure 1 SEM of IM-5
3.2 XRD
Figure 2 shows the XRD spectra of the parent and modifi ed IM5 samples. All the modi fi ed samples exhibited approximately the same peak position as the untreated IM-5 except for the lower intensity of peaks at around 8°—9°. Victor Abdelsayed, et al.[19]reported that the intensity of the diffraction peaks between 2θ=8°—9°significantly decreased after the introduction of Zn species and they ascribed their observation to the strong interaction between the FAl (Al framework) on the catalyst surface and Zn species. Moreover, no new peaks in the XRD spectra could be observed, indicating to good dispersion of Zn or Si species on the zeolite surfaces.
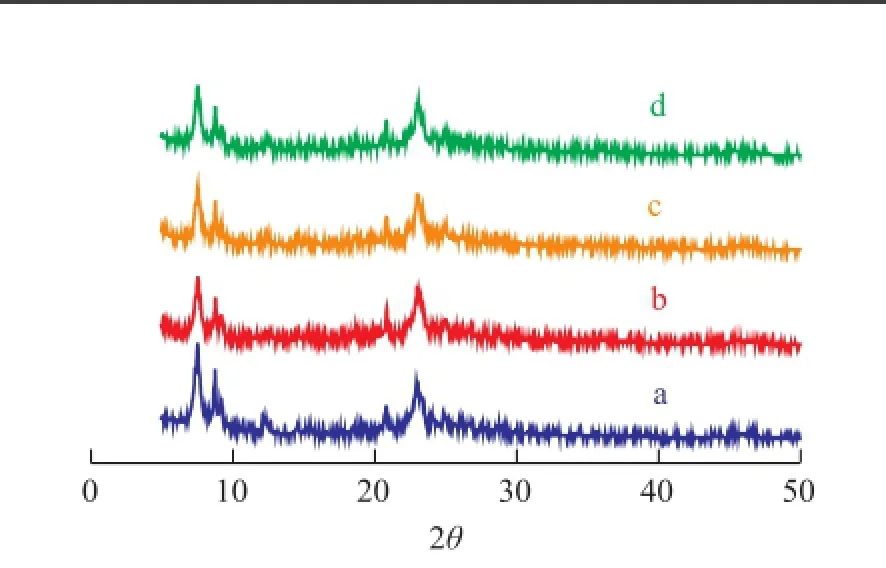
Figure 2 XRD spectra of the parent and modi fi ed IM5 samples
3.3 BET
It can be seen from Table 1 that the Zn-modi fi ed zeolite had a smallerSBETandVmicrocompared with the parent IM5 zeolite. And these two parameters decreased further with the growing introduction of Si species. The decrease in theVmicroassociated with the introduction of Zn species indicates that a fraction of the Zn phase existed in the micropores of the zeolite. The change brought forth by the addition of Si species is likely owing to the strong interaction between Si species and the external acid sites, which could block some of its pore structures. Besides,Vmesoremained unchanged after the modi fi cation with Zn or Si species.
3.4 NH3-TPD
The NH3-TPD curves of the raw and the silylated Zn-IM5 are shown in Figure 3. Silylation of Zn-IM5 led to a reduction in the concentration of both the weak and the strong acid sites. The band representing strong acid sites was invisible distinctly after the silylation. In addition, a lower temperature peak shift could be observed in the weak acid sites for Zn-Si-IM5, indicating that the weak acidity became even weaker after silylation.
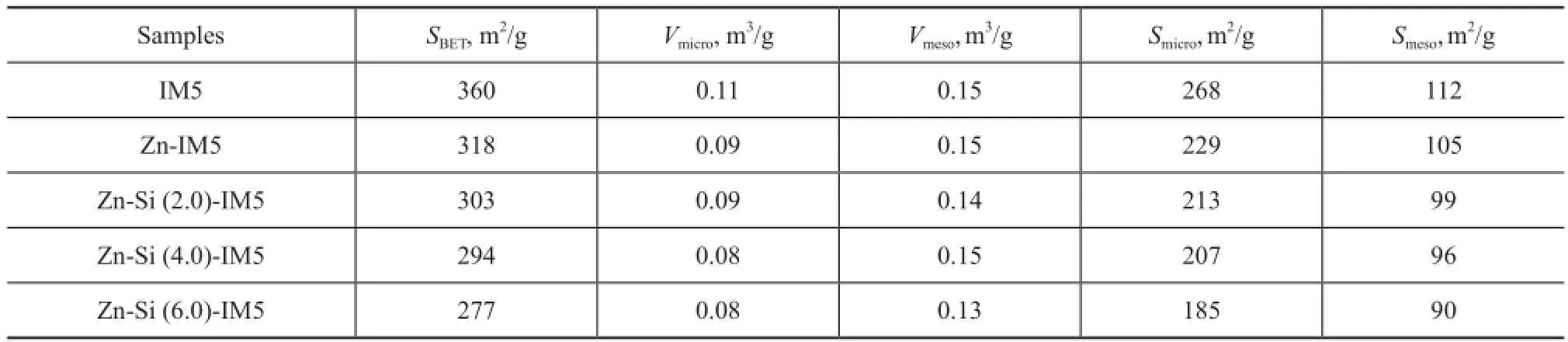
Table 1 Textural property of the catalyst
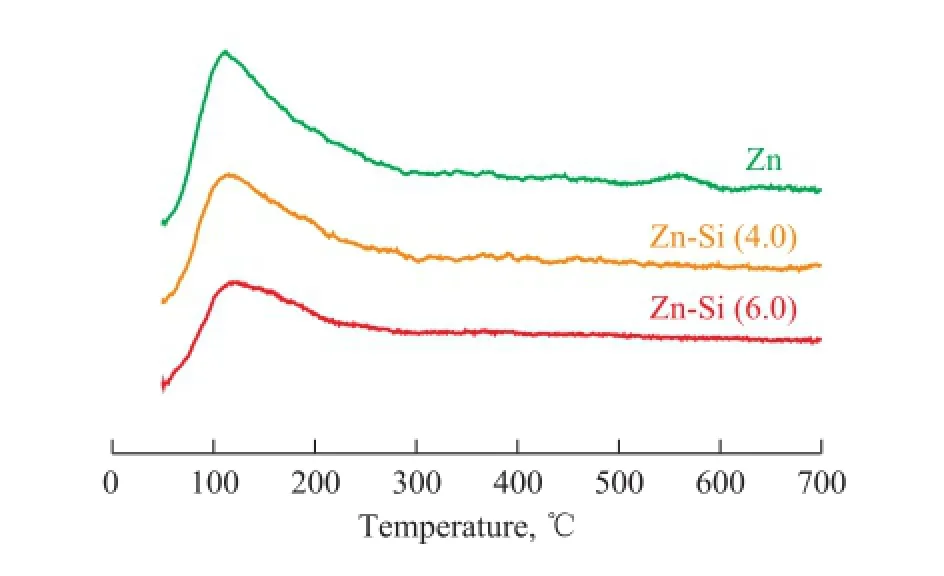
Figure 3 NH3-TPD of the Zn-IM5 and Zn-Si-IM5 samples
3.5 Coll-IR
Figure 4 presents the Coll-IR profiles of the Zn-IM5 and Zn-Si-IM5 samples. Areas of peaks on Figure 4(a) decreased gradually with the content of SiO2increasing from 2.0% to 6.0%, as a consequence of the reduction of acid sites on the external surface. It can be estimated from Figure 4(b) that the strong acid sites located on the external surface reduced sharply after silylation, and disappeared completely when the SiO2loading reached 4.0%. The observation of Coll-IR was in accordance with the NH3-TPD analysis.
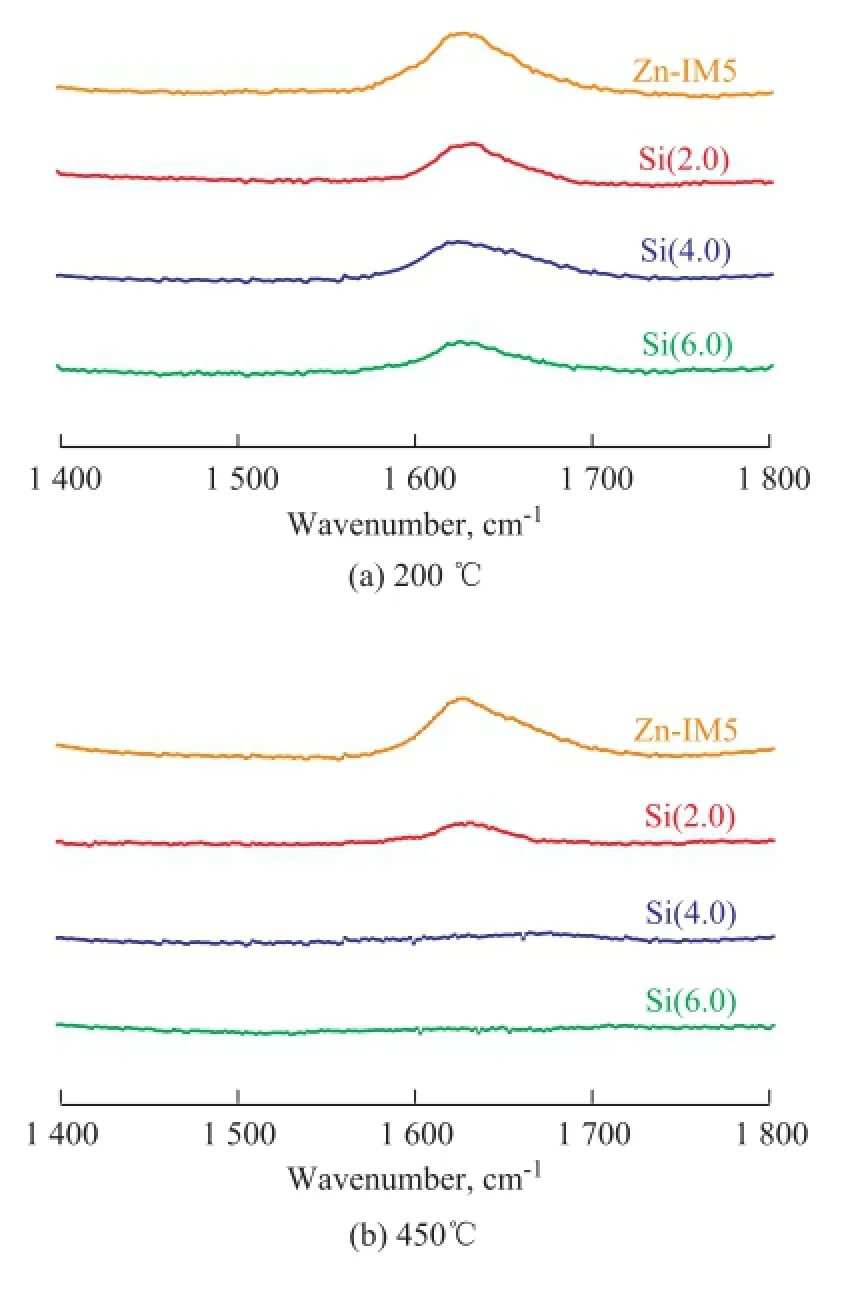
Figure 4 2,4,6-Collidine infrared spectra for Zn-IM5 and Zn-Si-IM5
3.6 TG
A universally accepted point is that coking happens preferentially on the acid sites, especially the strong acid sites. Since the addition of Si species could achieve such a reduction of the acidity (as shown in Figure 3 and Figure 4), the amount of coke on the coked catalysts was measured by TG. As shown in Figure 5, the coke deposits on the catalyst distinctly decreased after the introduction of Si species, and the result agreed well with the change of the acidity.
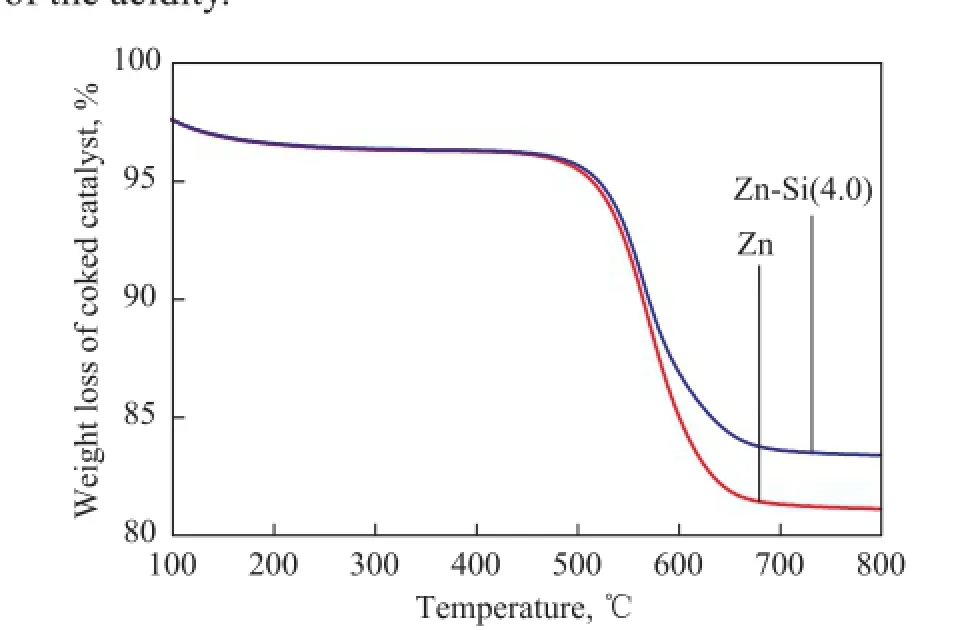
Figure 5 TG pro fi les of the coked Zn-IM5 and Zn-Si(4.0)-IM5 catalyst samples
3.7 Aromatization of butane
3.7.1 Effects of silylation on Zn-IM5
The conversion of butane and selectivity to BTX on Zn-IM5 and Zn-Si(4.0)-IM5 are shown in Figure 6. It can be observed that the Zn-Si(4.0)-IM5 catalyst was deactivated at a slightly lower rate as compared with Zn-IM5, denoting that the Zn-Si(4.0)-IM5 possessed a better stability. And the selectivity to BTX for Zn-Si-IM5 was higher at all the TOS studied in comparison with Zn-IM5.
With the help of NH3-TPD and Coll-IR, it is obvious that there were more acid sites on Zn-IM5 as compared with the silylated sample. Many side-reactions such as cracking could happen on strong acid sites, resulting in a high conversion. And this can be the reason of the high content of C1—C3generated on Zn-IM5, as shown in Table 2.
For the silylated sample, the reduction of acid sites and accordingly, the decrease in coke formation on the cata-lyst made the conversion reduced at a slightly lower rate than Zn-IM5 did.
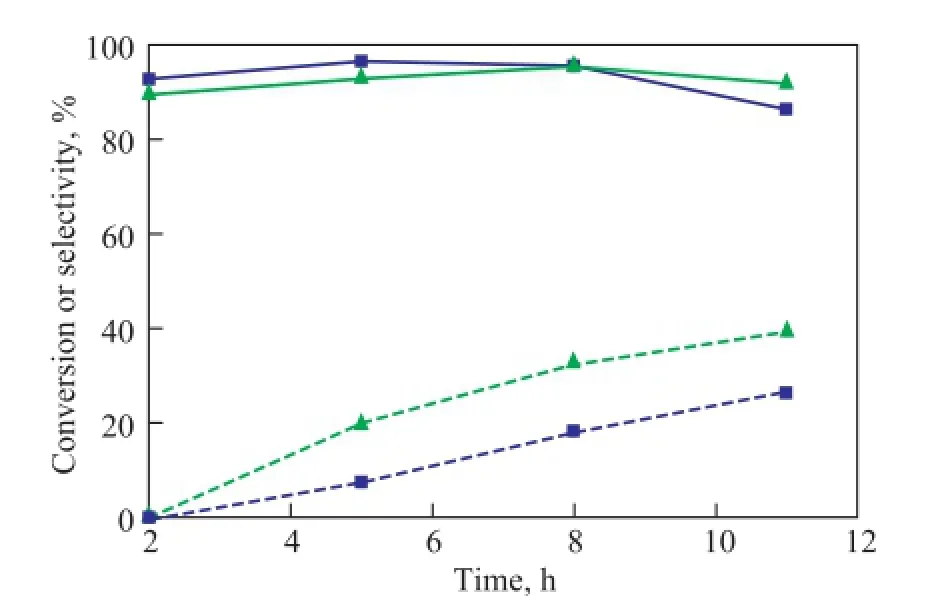
Figure 6 Aromatization of butane on Zn-IM5 and Zn-Si(4.0)-IM5:(Conversion (solid line); Selectivity to BTX (dot line).)
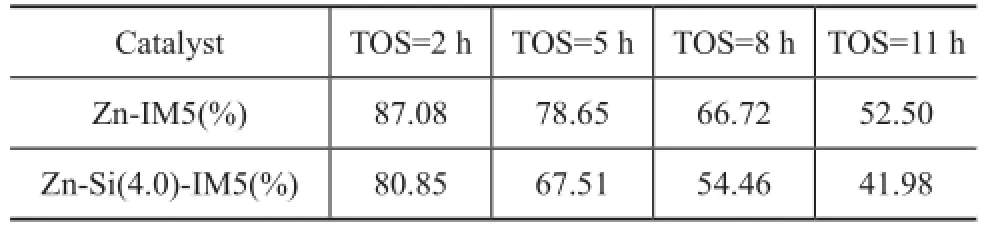
Table 2 Selectivity of C1—C3on Zn-IM5 and Zn-Si(4.0)-IM5
With the TOS going on, conversion on Zn-IM5 decreased owing to the increasing coverage of acid sites by coke. As for the silylated sample, the conversion turned out to be higher than that of Zn-IM5, since the loss of acid sites and the amount of coke deposits were much less.
The product distribution over Zn-IM5 and Zn-Si(4.0)-IM5 at TOS=7—9 h (when the conversion over the two catalysts was almost the same) is summarized in Table 3. The major reaction products included C1—C3alkanes (cracking products), alkenes, BTX and C9+(produced via a further reaction of light aromatics and oligomers on acid sites). Compared with Zn-IM5, the BTX yield was higher on Zn-Si-IM5 at the expense of C1—C3alkanes and C9+ formation.
Conclusions can be drawn from the combination of catalytic activity with characterization that the increase of BTX yields mainly benefits from two aspects. On one hand, the introduction of Si species narrows the pores and shifts its dimension more suitable for aromatics. On the other hand, deposition of Si-species reduces the acidity of the sample. The deactivation of acid sites due to the silylation impairs the activity of cracking and the further reaction of oligomers which are responsible for the formation of C1—C3alkanes and C9+, respectively.
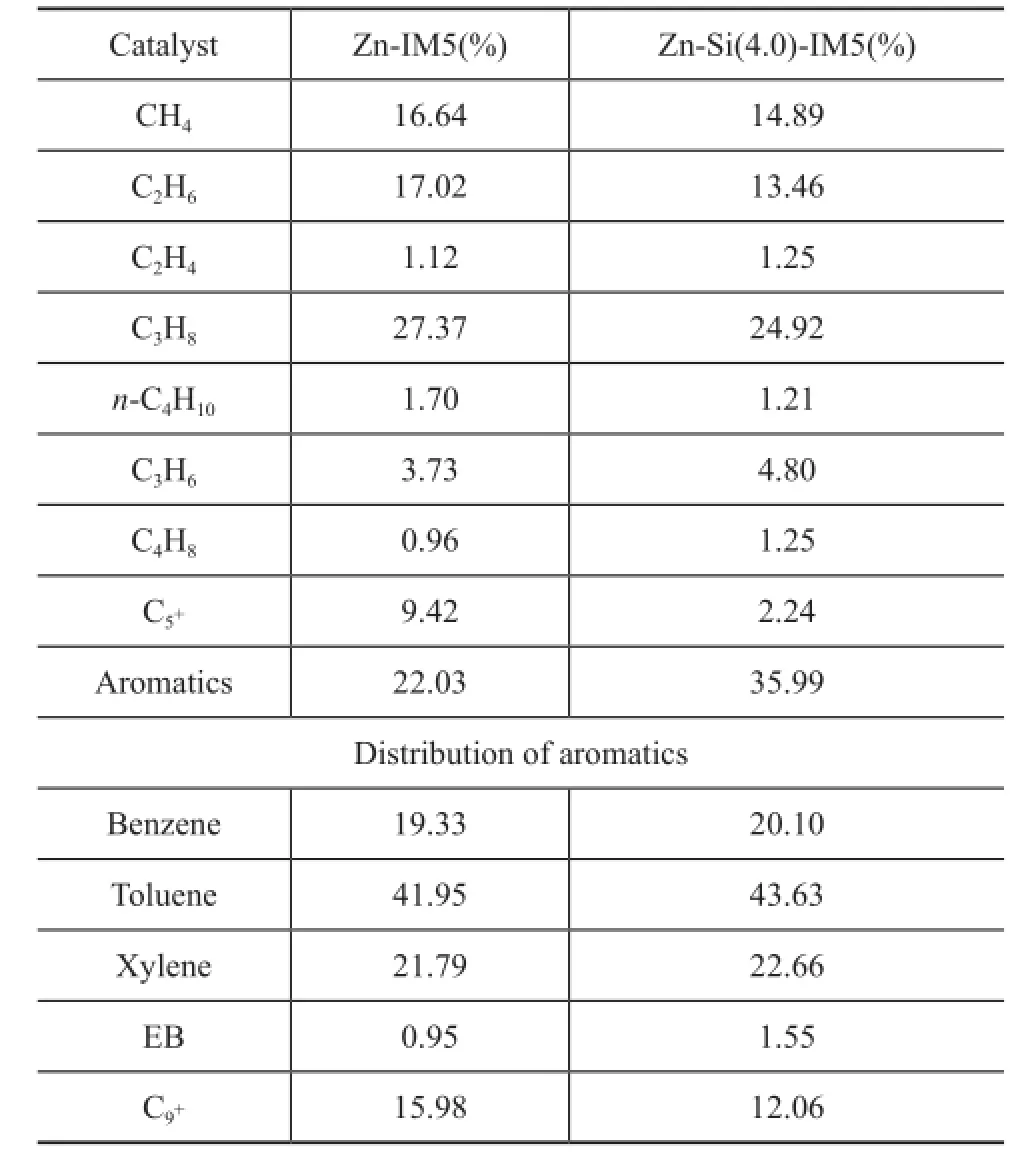
Table 3 The distribution of products on Zn-IM5 and Zn-Si(4.0)-IM5
3.7.2 In fl uence of silylation temperature
Figure 7 shows that as temperature increased from 40 ℃to 80 ℃, the yield of BTX increased initially, and then decreased. The best catalytic activity was achieved by the sample that was silylated at 50 ℃. The observed change in the catalytic activity could be ascribed to the hydrolysis of TEOS which would be strongly affected by the temperature.
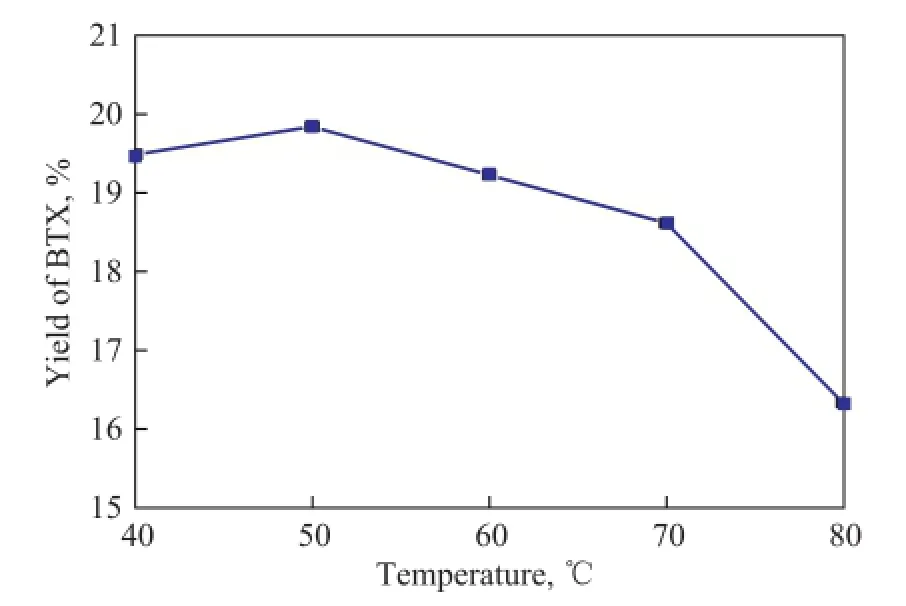
Figure 7 In fl uence of silylation temperature on the BTX yield
On one hand, if the silylation process was carried out at a relatively low temperature, the hydrolysis of TEOS would be incomplete, because the hydrolysis rate was going to be slow. On the other hand, if the temperature was too high,the hydrolysis would take place at a too fast rate to make the Si species dispersed well on the external surface.
3.7.3 In fl uence of the Si content
It can be seen from Figure 8 that the yield of BTX at fi rst reached a maximum and then decreased with further silylation. The highest yield was obtained on the sample containing 4.0% of SiO2. According to Figure 3 and Figure 4, it seems likely that the shortage of acidity would be the reason for the low activity on the samples containing 6.0% of SiO2and 8.0% of SiO2, respectively. It can be seen that there were almost no strong acid sites, and the weak acid sites turned out to be much less as compared with the sample containing 4.0% of SiO2. So, it looks like that the catalytic activity was decreased upon further silylation, since the acidity of catalyst sample decreased too much to satisfy the demand of reaction.
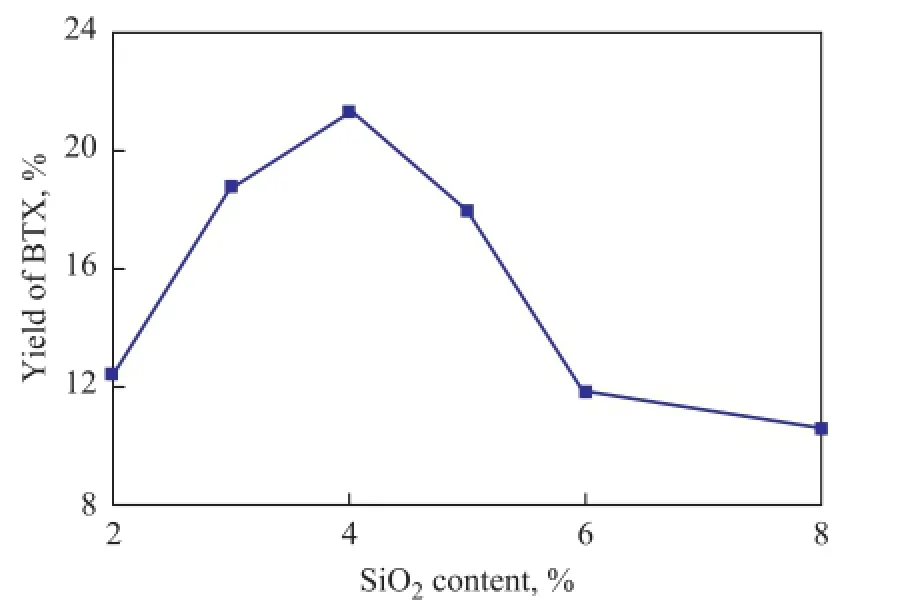
Figure 8 In fl uence of Si content on the yield of BTX
4 Conclusions
The in fl uence of silylation on the pore structure, acidity and catalytic activity of aromatization was investigated. The effect of silylation could clearly decrease the acidity and narrow the pores of the catalyst sample, thereby making the catalyst more suitable for the reaction. It was found out that the catalyst modi fi ed by Si species showed a high selectivity to the desirable products at the expense of the formation of light alkanes and C9+. The best treatment way was found to cover a silylation temperature of 50 ℃ and a SiO2loading of 4.0%.
Acknowledgement: This work is fi nancially supported by the Opening Project of the State Key Laboratory of Catalytic Material and Reaction Engineering of Sinopec Research Institute of Petroleum Processing. (No. 33600000-14-ZC0607-0010).
[1] Wang Linsheng, Tao Longxiang, Xie Maosong, et al. Dehydrogenation and aromatization of methane under non-oxidizing conditions[J]. Catalysis Letters, 1993, 21(1): 35-41
[2] Montes A, Giannetto G. A new way to obtain acid or bifunctional catalysts. V. Considerations on bifunctionality of the propane aromatization reaction over [Ga, Al]-ZSM-5 catalysts[J]. Applied Catalysis A: General, 2000, 197(1): 31-39
[3] Le van Mao R, Yao Jianhua, Dufresne L A, et al. Hybrid catalysts containing zeolite ZSM-5 and supported gallium oxide in the aromatization of n-butane[J]. Catalysis Today, 1996, 31(3/4): 247-255
[4] Martindale D C, Kuchar P J, Olson R K. Aromatics from LPG using the Cyclar process[C]//Proceeding of AIChE Summer National Meeting, Denver, Colorado, 1988
[5] Zhang Hengqiang, Kong Aiguo, Ding Yongjie, et al. Lowtemperature activation of methane over rare earth metals promoted Zn/HZSM-5 zeolite catalysts in the presence of ethylene[J]. Journal of Natural Gas Chemistry. 2011, 20(3): 243-248
[6] Lubango L M, Scurrell M S. Light alkanes aromatiation to BTX over Zn-ZSM-5 catalysts: Enhancements in BTX selectivity by means of a second transition metal ion[J]. Applied Catalysis A: General 2002, 235(1): 265-272
[7] Fan Jinlong, Xu Lei, Xu Yarong. Study of aromatization reactivity of post-MTBE C4 on ZSM-5 catalyst[J]. Petroleum Processing and Petrochemicals, 2015, 46(3): 15-17 (in Chinese)
[8] Zhang Ruojie, Wan Hai, Zhang Le, et al. Synthesis of bimetal ZSM-5/L composite zeolite and its catalytic performance for aromatization reaction[J]. Petroleum Processing and Petrochemicals, 2015, 46(7): 67-72 (in Chinese)
[9] Tempelman C H L, de Rodrigues V O, van Eck E R H, et al. Desilication and silylation of Mo/HZSM-5 for methane dehydroaromatization[J]. Microporous and Mesoporous Materials, 2015, 203: 259-273
[10] Tempelman C H L, Hensen E J M. On the deactivation of Mo/HZSM-5 in the methane dehydroaromatization reaction[J]. Applied Catalysis B: Environmental, 2015, 176-177: 731-739
[11] Benazzi E, Guth J L, Rouleau L. IM-5 zeolite, method of preparation and catalytic applications thereof: WO 98/17581[P]. 1998
[12] Corma A, Martinez-Triguero J, Valencia S, et al. IM-5: a highly thermal and hydrothermal shape-selective cracking zeolite[J]. J Catal, 2002, 206(1): 125-133
[13] Lee S H, Lee D K, Shin C H, et al. Synthesis, characterization, and catalytic properties of zeolites IM-5 and NU-88[J]. J Catal, 2003, 215(1): 151-170
[14] Liu Heng, Wu Shujie, Guo Ye, et al. Synthesis of Mo/ IM-5 catalyst and its catalytic behavior in methane nonoxidative aromatization[J]. Fuel, 2011, 90(4): 1515–1521
[15] Koji Nishi, Shin-ichi Komai, Kazumi Inagaki, et al. Structure and catalytic properties of Ga-MFI in propane aromatization[J]. Applied Catalysis A: General, 2002, 223(1): 187-193
[16] János H, Zoltán K, ágnes F, et al. Indium and gallium containing ZSM-5 zeolites: acidity and catalytic activity in propane transformation[J]. Catalysis Today, 1996, 31(3): 293-304
[17] Zheng Shourong, Heydenrych H R, Jentys A, et al. In fl uence of surface modi fi cation on the acid site distribution of HZSM-5[J]. Journal of Physical Chemistry B, 2002, 106(37): 9552-9558
[18] Song Wen, Liu Zhiting, Liu Liping, et al. A solvent evaporation route towards fabrication of hierarchically porous ZSM-11 with highly accessible mesopores[J]. RSC Advances, 2015(5): 31195-31204
[19] Abdelsayed V, Shekhawat D, Smith M W. Effect of Fe and Zn promoters on Mo/HZSM-5 catalyst for methane dehydroaromatization[J]. Fuel, 2015, 139: 401-410
Received date: 2015-08-19; Accepted date: 2015-11-27.
Prof. Meng Xuan, Telephone: +86-21-64252383; E-mail: mengxuan@ecust.edu.cn.
- 中國煉油與石油化工的其它文章
- Experimental Study of UDS Solvents for Purifying Highly Sour Natural Gas at Industrial Side-stream Plant
- Highly Active and Stable Ni2P/SiO2Catalyst for Hydrogenation of C9Petroleum Resin
- Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
- Investigation of Different Coke Samples Adhering to Cyclone Walls of a Commercial RFCC Reactor
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Preparation of Core-Shell Composite of Y@Mesoporous Alumina and Its Application in Heavy Oil Cracking

