A new species of genus Fejervarya (Anura: Dicroglossidae) from northern Thailand
Chatmongkon SUWANNAPOOM, Zhi-Yong YUAN, Nikolay A. POYARKOV Jr., Fang YAN, Somboon KAMTAEJA, Robert W. MURPHY, Jing CHE,*
1State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming Yunnan 650223, China
2Division of Fishery, School of Agriculture and Natural Resources, University of Phayao, Phayao 56000, Thailand
3College of Forestry, Southwest Forestry University, Kunming Yunnan 650224, China
4Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Mengla Yunnan 666303, China
5Department of Vertebrate Zoology, Biological Faculty, Lomonosov Moscow State University, Leninskiye Gory Moscow 119991, Russia
6Joint Russian-Vietnamese Tropical Research and Technological Center Under the A.N. Severtsov Institute of Ecology and Evolution RAS, South Branch, Ho Chi Minh City, Vietnam
7Faculty of Education, Chiang Rai Rajabhat University, Chiang Rai 57100, Thailand
8Centre for Biodiversity and Conservation Biology, Royal Ontario Museum, Toronto M5S 2C6, Canada
A new species of genus Fejervarya (Anura: Dicroglossidae) from northern Thailand
Chatmongkon SUWANNAPOOM1,2,4, Zhi-Yong YUAN3,4, Nikolay A. POYARKOV Jr.5,6, Fang YAN1,4, Somboon KAMTAEJA7, Robert W. MURPHY1,8, Jing CHE1,4,*
1State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming Yunnan 650223, China
2Division of Fishery, School of Agriculture and Natural Resources, University of Phayao, Phayao 56000, Thailand
3College of Forestry, Southwest Forestry University, Kunming Yunnan 650224, China
4Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Mengla Yunnan 666303, China
5Department of Vertebrate Zoology, Biological Faculty, Lomonosov Moscow State University, Leninskiye Gory Moscow 119991, Russia
6Joint Russian-Vietnamese Tropical Research and Technological Center Under the A.N. Severtsov Institute of Ecology and Evolution RAS, South Branch, Ho Chi Minh City, Vietnam
7Faculty of Education, Chiang Rai Rajabhat University, Chiang Rai 57100, Thailand
8Centre for Biodiversity and Conservation Biology, Royal Ontario Museum, Toronto M5S 2C6, Canada
We describe a new species of frog in the dicroglossid genus Fejervarya from Ban Monjong, Omkoi District, Chiang Mai Province, northern Thailand. Analysis of DNA sequence data from the mitochondrial gene 16S, advertisement calls, and morphological distinctiveness support recognition of the new species. Matrilineal genealogy suggests that the new population from Chiang Mai is a sister taxon to the South Asian clade that includes F. syhadrensis, F. granosa, and F. pierrei. The new species, Fejervarya chiangmaiensissp. nov., differs morphologically from its congeners by its relatively small body size and proportions and the presence of dorsal warts and dermal ridges. Discovery of this new species indicates that the biodiversity of amphibians in this region remains underestimated.
Phylogeny; Mitochondrial DNA; 16S rRNA; Chiang Mai Province; Cryptic species; Fejervarya chiangmaiensissp. nov.
INTRODUCTION
The cricket frogs Fejervarya Bolkay currently contain 40 species (Frost, 2016), most of which occur in East and Southeast Asia, to the Indian subcontinent, including Sri Lanka, and further to Pakistan, Nepal, and Bangladesh (Dinesh et al., 2015). This genus comprises two reciprocally monophyletic species groups: (1) South Asian group; and (2) East and Southeast Asian group (Dinesh et al., 2015). Seven species within these two groups occur in Thailand (Frost, 2016), including Fejervarya andamanensis (Stoliczk, 1870); Fejervarya cancrivora (Gravenhorst, 1829); Fejervarya limnocharis (Gravenhorst,11829); Fejervarya moodiei (Taylor, 1920); Fejervarya multistriata (Hallowell, 1861); Fejervarya orissaensis (Dutta, 1997); and, Fejervarya triora (Stuart et al., 2006). Except for F. andamanensis, which assigns to the South Asian group, all other Thai species assign to the East and Southeast Asian group (Frost, 2016). Several molecular studies have suggested that F. limnocharis from this region might represent an unnamed species (Dinesh et al., 2015; Kotaki et al., 2010; Kuramoto et al., 2007), such as, F. sp. hp3 from Pilok, Thailandand F. sp. hp2 from Bankok, Thailand (Kotaki et al., 2010). However, morphological characteristics of specimens from these regions have not been examined in detail. A closer inspection of many of these species is necessary to better understand and effectively manage the amphibian biodiversity in Thailand.
Our recent fieldwork in Thailand resulted in the discovery of a new population of Fejervarya. To clarify its phylogenetic relationships with other species of Fejervarya, we reconstructed a matrilineal genealogy for the genus using mitochondrial DNA (mtDNA) sequence data from the ribosomal RNA gene 16S, which is widely recognized as a useful genetic marker for amphibian systematics (Vences et al., 2005a, b). Our genealogy provides evidence for the phylogenetic placement of the new species. In addition, we examined the major morphological characters and acoustic data traditionally used in dicroglossid frogs. Based on these data, we describe the population as a new species of Fejervarya.
MATERIALS AND METHODS
Sampling
Fieldwork was conducted in the vicinity of Monjong village, Omkoi District, Chiang Mai Province, Thailand (Figure 1) from June to September 2013. Twelve adult male frogs were collected in the field and photographed in situ. Specimens were euthanized using benzocaine after extraction of liver tissue, which was stored in 95% ethanol. The voucher specimens were fixed with 10% formalin and later stored in 70% ethanol. All specimens were deposited in the herpetological collection of the Museum of the Kunming Institute of Zoology (KIZ), Chinese Academy of Sciences (CAS). The protocols for collection of specimens in this study were approved by the Animal Ethics Committee of the KIZ, CAS.
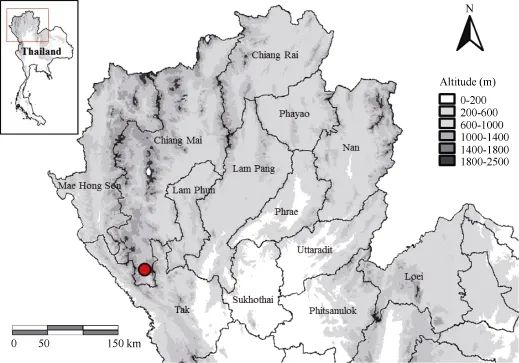
Figure 1 Known distribution of Fejervarya chiangmaiensis sp. nov. from northern Thailand, Omkoi District, Chiang Mai Province (red cycle=type locality)
Molecular methods
Total genomic DNA was extracted from tissue samples using standard phenol-chloroform protocols (Sambrook et al., 1989). One fragment of mtDNA encompassing the 16S rRNA gene (16S) was amplified using primers 16Sar: 5'-CGCCTGTTTAYC AAAAACAT-3' and 16Sbr: 5'-CCGGTYTGAACTCAGATCAY GT-3' from Kocher et al. (1989). Amplification was performed in a 25 μL volume reaction with the following procedure: initial denaturation at 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. The PCR products were purified with a Gel Extraction Mini Kit (Watson Biotechnologies, Shanghai, China). All sequencing was conducted on a ABI PRISM 3730 automated sequencer (Applied Biosystems, Foster City, CA, USA) at KIZ, CAS. All individuals were sequenced in both directions.
Phylogenetic analysis
Sequences were examined for quality of signal and confirmed for complementarity using DNASTAR 5.0 (DNASTAR Inc., Madison, WI, USA). As the very small body size of the new specimens was most similar to that of species from the South Asian Fejervarya group, we chose F. limnocharis, F. triora, and Limnonectes fujianensis as outgroups based on Kotaki et al. (2008, 2010). Available sequences for these species were downloaded from GenBank as outgroups (Table 1). All mtDNA sequences were aligned using MUSCLE (Edgar, 2004).
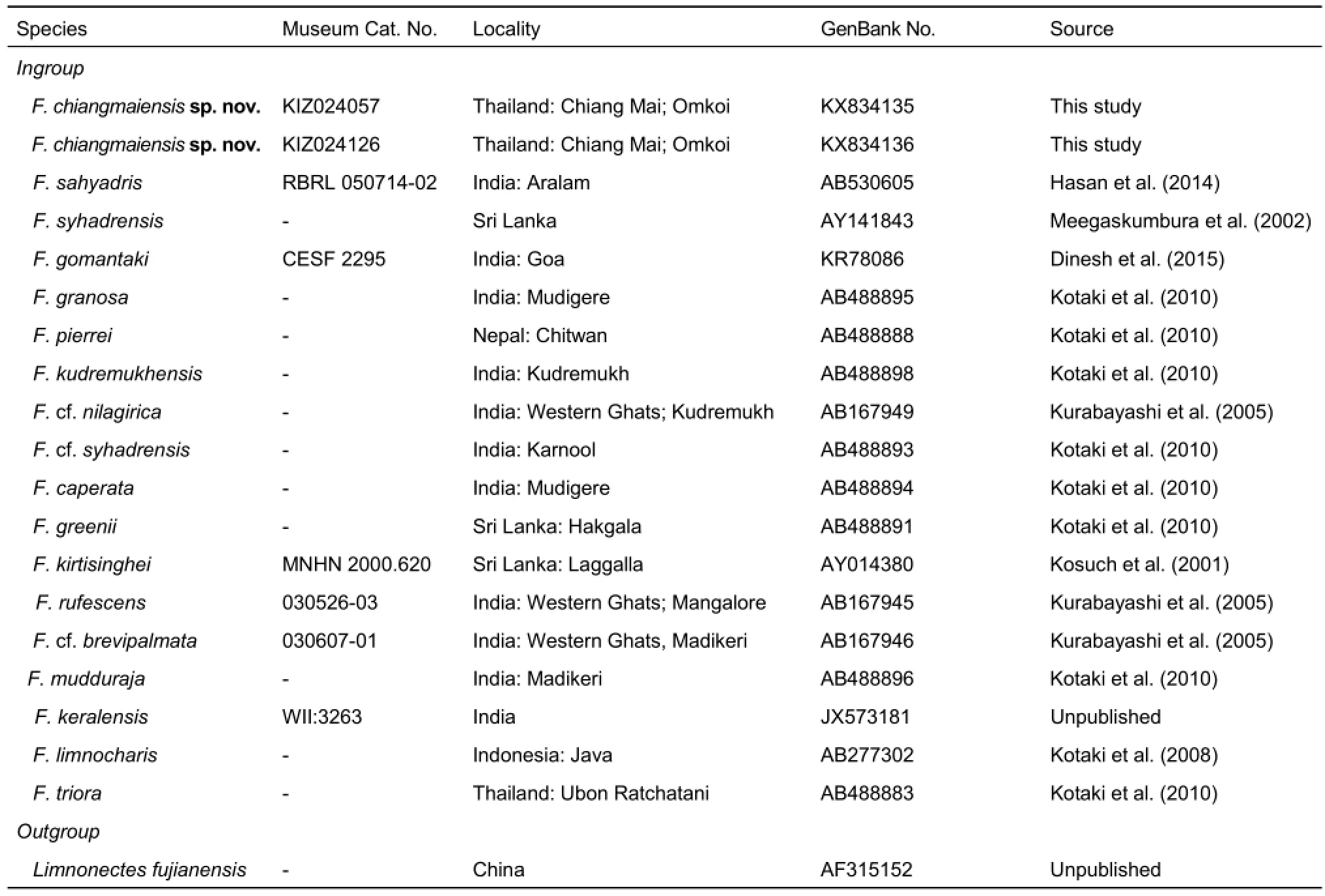
Table 1 Specimens corresponding to genetic samples included in phylogenetic analysis, their localities, and GenBank accession numbers
Phylogenetic reconstructions were executed using maximum parsimony (MP), maximum likelihood (ML), and Bayesian inference (BI). Character-based MP analyses were conducted using PAUP* v4.0b10 (Swofford, 2003). Full heuristic tree searches with tree bisection-reconnection were executed for 1000 replications. Bootstrap support (BS) for the MP tree involved 1 000 pseudoreplicates (Felsenstein, 1985). The ML analyses were performed with RAxML v7.0.4 (Stamatakis et al., 2008) using the Gamma model of rate heterogeneity option. Nodal support was estimated using 1 000 BS pseudoreplicates. For BI, the best-fit model of DNA sequence evolution was chosen using MrModeltest v2.3 (Nylander, 2004) under the Akaike information criterion. The GTR+I+G model was selected and used to generate a BI tree using MrBayes v3.1.2 (Ronquist & Huelsenbeck, 2003). Analyses were run for five million generations using four chains while sampling one of every 1000 tree generations and discarding the fi rst 25% as burn-in. Loglikelihood scores were tracked to assure stationarity.
Genetic distances among taxa were calculated using the Kimura 2-parameter model in MEGA 5. The matrilineal genealogy was assumed to reflect the phylogenetic relationships of the species. We considered tree nodes with bootstrap values 70% or greater and posterior probabilities values over 0.95 as sufficiently resolved, those between 75% and 50% (0.95 and 0.90 for BI) as tendencies, and those below 50% (0.90 for BI) as non-resolved (Huelsenbeck & Hillis, 1993).
Morphology
Measurements were made with digital calipers to the nearest 0.1 mm. Twenty morphometric characters of post metamorphic individuals were as per Matsui (1984) as follows: (1) snout-vent length (SVL); (2) head length (HL); (3) snout-nostril length (SNL); (4) nostril-eye length (N-EL); (5) snout length (SL); (6) eye length (EL); (7) tympanum-eye distance (T-ED); (8) head width (HW); (9) internarial distance (IND); (10) interorbital distance (IOD); (11) upper eyelid width (UEW); (12) forelimb length (FLL); (13) lower arm length (LAL); (14) first finger length (FFL); (15) hindlimb length (HLL); (16) tibia length (TL); (17) foot length (FL); and (18) inner metatar sal tubercle length (IMTL). Additionally, we also measured (19) finger length (I-IV FL) and (20) toe length (I-V TOEL). Toe-webbing states followed Savage (1975).
We obtained comparative morphological data from museum specimens (Table 3), photographs of these specimens in life,and previously published literature: Jerdon, 1853; Günther, 1859, 1869; Peters, 1871; Boulenger, 1905; Annandale, 1919; Rao, 1922, 1937; Smith, 1930; Taylor, 1962; Inger, 1966; Dubois, 1975, 1984; Pillai, 1979; Manamendra-Arachchi & Gabadage, 1996; Dutta, 1997; Manthey & Grossmann, 1997; Dubois et al., 2001; Stuart et al., 2006; Orlov & Ananjeva, 2007; Matsui et al., 2007; Kuramoto et al., 2007; Ohler et al., 2009; Djong et al., 2011; Howlader, 2011a, b; and Purkayastha & Matsui, 2012. Due to the high level of cryptic diversity within Fejervarya, we relied on examination of topotypic material and/or original descriptions of species when available.
Acoustics
Advertisement calls of the newly collected population were recorded in situ on 30 June, 2013, from 2200h to 2330h using a digital recorder (EDIROL R-09, Roland, Swansea, UK) with built-in microphone (frequency responses of 20–22.000 Hz). Files were recorded as 16-bit WAV files at a sampling frequency of 44.1 kHz and 22 advertisement calls from a single individual were recorded. The ambient temperature (26 °C) was measured with a digital thermometer immediately after recording. Total duration of the recording was 3.0 s. We generated sound spectrograms of all field recordings using Syrinx-PC sound analysis software (J. Burt, Seattle, WA, USA) with the following settings: FFT size 512 samples and Hanning FFT window for spectrograms and power spectra, with FFT samples overlapping 75% for spectrograms. Comparative advertisement call characters for dicroglossids were taken from Kuramoto et al. (2007), Ohler et al. (2009), and Purkayastha & Matsui (2012).
RESULTS AND DISCUSSION
Morphological measurements and variations are summarized in Table 3. This species had both short and long calls, though the latter were not always emitted (Figure 2). The shorter call consisted of a series of pulsed notes. Each of these notes lasted 3.0±0.4 s and was composed of 9-12 pulses/call (average 11.2±1.8). The note interval was 1.81±0.598 s, the dominant frequency was 2.0±0.03 kHz, and the second harmonic was about 3 857±0.036 kHz. The call had a slight frequency modulation.
All unique de novo sequences were deposited in GenBank under accession numbers KX834135 and KX834136. Sequencing generated a total of 700 base pairs (bp) of 16S rRNA data, among which 496 positions were potentially parsimony-informative. The ML, MP, and BI analyses produced similar topologies. Monophyly of the South Asian group of Fejervarya was recovered (Figure 3). Within these frogs, 16 species occurred in strongly supported matrilines A and B (Figure 3). Matriline A contained seven matrilines A1-7 (Figure 3), but their relationships were generally poorly resolved. Submatriline A1 contained the new population from Chiang Mai, which was the sister group of sub-matriline A2, which included F. syhadrensis, F. granosa, and F. pierrei. Fejervarya sahyadris formed a sister relationship with F. cf. syhadrensis within submatriline A3. Sub-matriline A4 included F. kudremukhensis and F. cf. nilagirica. Sub-matriline A5 included F. caperata. Submatriline A6 included F. greenii and F. kirtisinghei. Sub-matriline A7 included F. rufescens. Matriline B was comprised of F. brevipalmata, F. murthii, and F. keralensis.
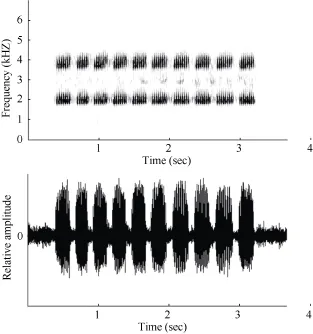
Figure 2 Sonogram and oscillogram of a Fejervarya chiangmaiensis sp. nov. call recorded on 30 June, 2013, at an agricultural farm in Ban Monjong, Omkoi District, Chiang Mai Province, Thailand
The genetic distances among the 13 South Asian species ranged from 0.4% to 14.8% (Table 2). Interspecific genetic distances between the newly discovered Chiang Mai matriline A1 and members of sub-matriline A2 from Sri Lanka, India, and Nepal ranged from 6.0% to 6.7%. The new species differed from F. cf. syhadrensis and F. sahyadris (both within matriline A2) from India by 8.8% and 9.0%, respectively. The new species differed from F. caperata from India (matriline A4) by 9.2%, F. kudremukhensis and F. nilagirica (matriline A5) from India by 9.0%, F. greenii and F. kirtisinghei from Sri Lanka (matriline A6) by 9.7% and 9.8%, respectively, and F. rufescens from matriline A7 by 11.3%. High genetic diversity between the Chiang Mai specimens and the other South Asian matrilines suggest it could be a new species, which we describe below.
Fejervarya chiangmaiensis sp. nov. (Figure 4,5)
Holotype.Adult male (KIZ024057), from Ban Monjong, Omkoi District, Chiang Mai Province, Thailand (N17°28'16.93", E98°27'28.26", 460 m a.s.l.), collected by Chatmongkon Suwannapoom on 30 June, 2013.
Paratypes.Eleven males KIZ024053–56, KIZ024058, KIZ024126, KIZ024096–100; collected by Chatmongkon Suwannapoom, Zhiyong Yuan, and Fang Yan; other data same as the holotype.

Figure 3 Matrilineal relationships among species of Fejervarya inferred from mtDNA 16S rRNA
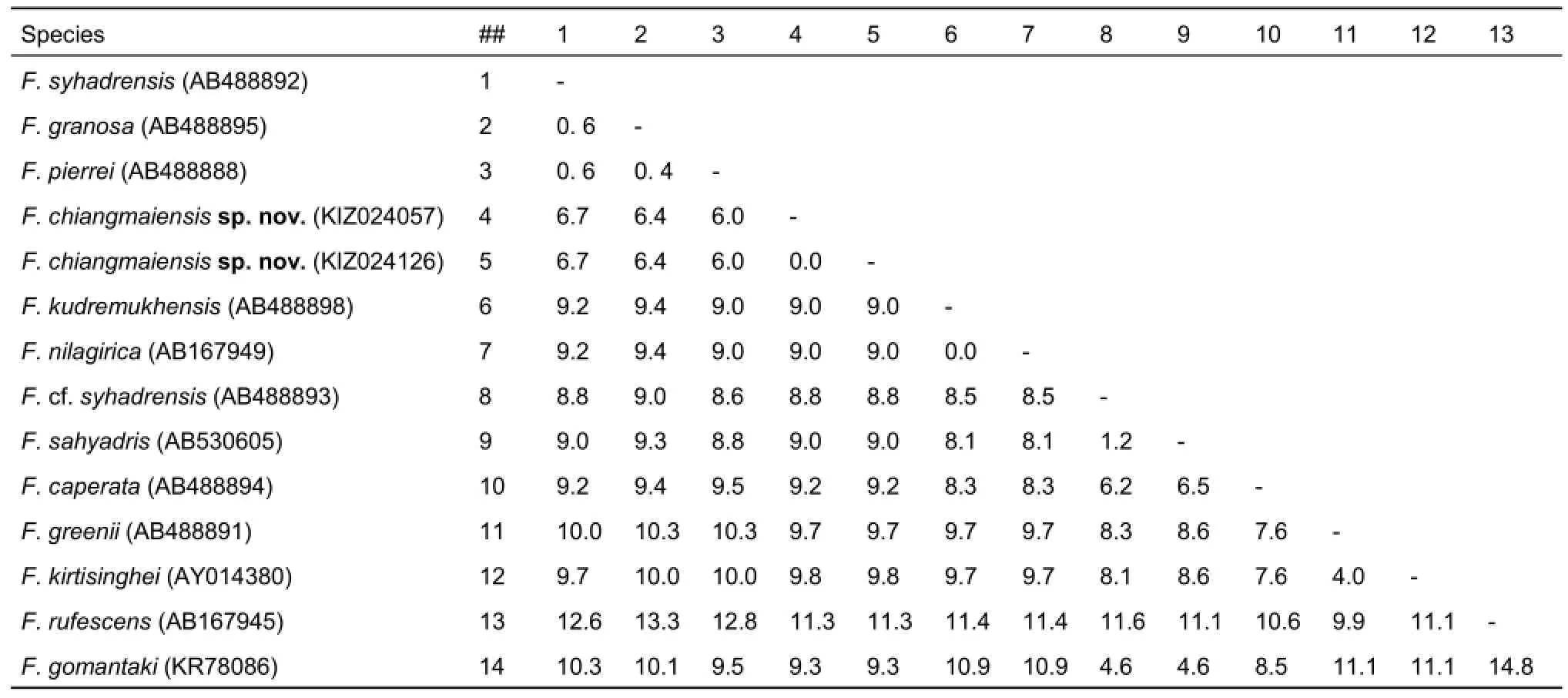
Table 2 Matrix of uncorrected K2P distances among partial 16S rRNA gene sequences of members of Fejervarya (GenBank accession numbers follow species names)
Diagnosis.The new species assigns to Fejervarya on the basis of its position in the matrilineal phylogeny (Figure 3) and the following morphological characteristics: (1) slightly pointed snout; (2) comparatively poorly developed foot webbing; (3) lateral line system in adult absent; (4) characteristic “Fejervarya”-lines present; (5) femoral glands absent; (6) tympanum comparatively small; and (7) tibia length slightly more than a half of SVL. The new species is characterized by a combination of the following morphological characteristics: (1) small-size (males mean SVL 26.3-29.1 mm; n=12) (Table 3); (2) head length greater than head width; (3) tympanum small, discernible but unclear; (4) slightly elongated cylindrical internal metatarsal tubercle; (5) relative finger length (from longest to shortest) when addressed: II<IV<I<III; (6) webbing formula on foot=I 1-2 II1-2? III 2-3 IV 3-1 V; (7) cream vertebral line usually present medially lasting from between the eyes to the vent; males with paired dark vocal sacs (Figure 4); (8) dorsal and lateral parts of head and body, including body flanks, shagreened; posterior part of dorsum with distinct, elongate, glandular warts, continuing on dorsal surface of legs and arms; (9) dorsal skin showing rare, small, longitudinal folds arranged in series; and (10) advertisement call consisting of a long series of partially pulsed notes, each of which lasts 3.0±0.4 s, with 9-12 pulses/call (average 11.2±1.8), note interval of 1.8±0.6 s, and dominant frequency of 2.0 kHz.

Figure 4 Dorsal (A) and ventral views (B) (scale bar, 10 mm); Ventral views of the right hand (C) and foot (D) (scale bar, 2 mm) of the male holotype of Fejervarya chiangmaiensis sp. nov. (KIZ024057) after preservation

Figure 5 Life photo of Fejervarya chiangmaiensis sp. nov. in situ; calling adult male paratype KIZ024056 from type locality
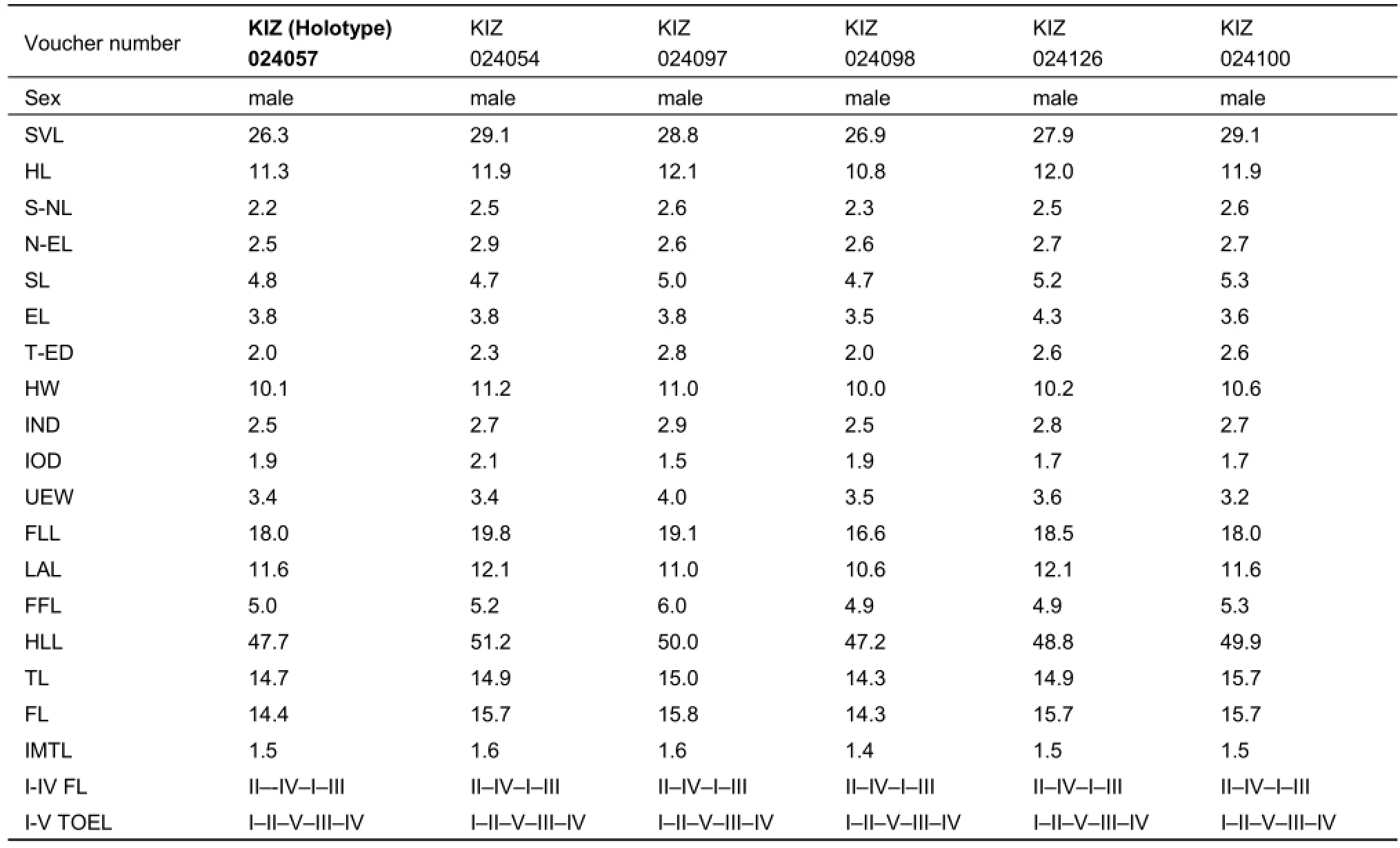
Table 3 Measurements of type specimens (mm) (n=12 in males) of Fejervarya chiangmaiensis sp. nov.
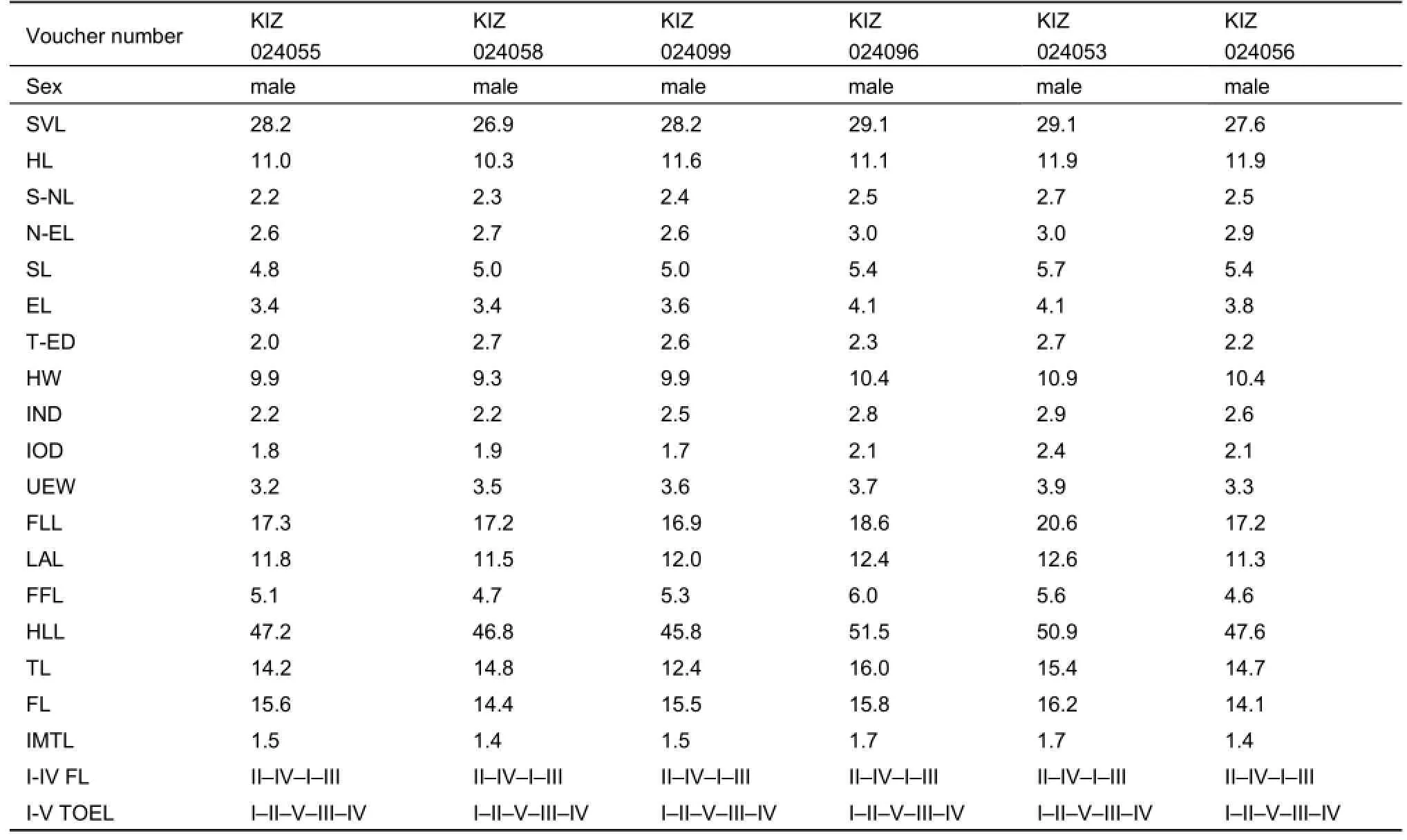
Continued
Description of holotype.(all measurements in mm; see Table 3): Adult male (KIZ024057; Figure 4). Small-sized frog specimen with SVL=26.3 mm, body habitus moderately stout (Figure 4A).
Head.Head of moderate size, head longer (HL 11.3 mm) than wide (HW 10.1 mm; HW/HL ratio 0.9), convex (Figure 4A). Snout more or less pointed from above; length (SL 4.8 mm) longer than horizontal diameter of eye (EL 3.8 mm) and interorbital distance (IOD 1.9 mm). Snout relatively distinct, protuberant; loreal region concave, angle to upper surface of snout rather vertical; canthus rostralis not sharp, rounded. Interorbital space slightly convex, much narrower (IOD 1.9 mm) than upper eyelid (UEW 3.4 mm) and narrower than internarial distance (IND 2.5 mm). Nostrils rounded, with a distinct flap of skin laterally, nostril slightly closer to snout than to eye (S-NL: 2.2 mm; N-EL: 2.5). Eyes comparatively small, protuberant; EL 33.5% of HL; upper eyelid with minute granules, pupil horizontal. Tympanum (TYD 1.4 mm) visible, but poorly distinct, rounded; slightly more than the half of eye diameter (TD 53.0% of ED), tympanum-eye distance (TYE 2.0 mm) half its diameter. Pineal ocellus indistinct. Choanae triangular, rounded; vomerine ridge absent, vomerine teeth in two oblique lines between choanae, beginning at anterior border of choanae, slightly extending beyond its posterior border. Tongue rather large, cordate, notably forked with two projections at tip; median lingual process absent; tooth like projections on maxilla absent.
Forelimbs.Lower arm short, rather strong (LAL 11.6 mm), 64.3% of forelimb length (FLL 18 mm). Fingers short, thin, without dermal fringe; webbing absent, finger tips bluntly rounded and not enlarged to disks (Figure 4C). Relative finger lengths from shortest to longest: II<IV<I<III (I: 5.5 mm; II: 5.1 mm; III: 6.6 mm; IV: 5.5 mm). Subarticular tubercles prominent, rounded, single, all present; a single palmar (thenal) tubercle large, well-developed, rounded. Prepollex oval, distinct; supernumerary tubercles absent.
Hindlimbs.Hindlimbs comparatively long, HLL (47.7 mm), about 1.8 times that of SVL (26.3 mm). Tibia (TL 14.7 mm) slightly longer than femur and subequal to foot length (FL 14.4 mm). Toes long, thin, toe tips blunt, slightly rounded, not enlarged to disks (Figure 4D). Relative toe lengths from shortest to longest: I<II<V<III<IV (I: 5.90 mm; II: 7.46 mm; III: 10.71 mm; IV: 13.5 mm; V: 9.5 mm). Subarticular tubercles prominent, elongated ovalshaped, protuberant, simple, all present. Inner metatarsal tubercle prominent, long and slightly compressed laterally (IMT: 1.5), 4 times the length of toe I. Foot webbing small, webbing formula I 1-2 II 1-2? III 2-3 IV 3-1 V (Figure 4C,D). Dermal fringe along toe V absent. Inner tarsal ridge present, flat. Outer metatarsal tubercle present, prominent, elongated; supernumerary tubercles absent; tarsal tubercle absent.
Skin and skin glands.Snout smooth with rare indistinct dermal granules, nares with low dermal flaps, small tubercles on upper eyelid. Dorsal and lateral surfaces of head and body, including body flanks, shagreened, posterior part of dorsum with distinct, round glandular warts, continuing on dorsal surfaces of legs and arms; Dorsal skin showing rare, small,longitudinal dermal ridges arranged in series. Anterodorsal part of thigh, anal region, dorsal surface of tibia, and tarsus with small granules; lateral sides of body, ventral surfaces of body and limbs smooth. Lateral-dorsal folds absent; lateral line system absent; “Fejervarya”-line present; supra-tympanic fold distinct, running from the posterior corner of eye backwards and downwards towards the incursion of the forelimb. Discernable macroglands, in particular rictal gland, absent.
Male secondary sexual characters.White nuptial pas present. Vocal sacs present, unique subgular pouch appear subdivided when inflated (Figure 5); pair of rounded openings in posterior part of mouth floor. No other discernable male secondary characters.
Coloration of adult in life.Dorsal ground color varies from dark green to dark brown; lateral sides greyish, ventral surfaces of body, head, and limbs whitish-cream. Marginal sides of gular area dark-greyish to blackish forming an M-shaped band (when inflated, vocal sac looks blackish; Figure 5). Transverse blackish-green to dark brown bands present on the dorsal surfaces of the thigh, tibia, and tarsus (Figure 5). Dorsal surfaces of fingers and toes greyish with indistinct transverse bands. Three to four dark brownish irregular blotches on each side of the upper jaw. Lateral sides of body with irregular greygreenish and whitish blotches; dark greenish blotches get bigger towards the axilla and groin. Thin orange-yellowish middorsal vertebral stripe runs from the anterior part of the interorbital space to the vent. Numerous fused irregular spots on the posterior surface of thigh dark green with thin dark brown reticulations in-between. Tympanum unclear dark green with darker circle in center, lower part of tympanum greyish. Iris greenish-bronze with dark reticulation; pupil horizontal.
Coloration in preservative.In alcohol the pattern described above was not obviously changed, although was slightly faded (Figure 4A). The yellowish tint faded the most, with greenish colors on the dorsal surfaces appearing brownish or graybrownish (Figure 4A); the ventral sides look much lighter than in life (Figure 4B); the belly appeared whitish. Dorsum turned greyish brown with many large dark-gray to black spots, thin mid-dorsal stripe turned whitish, running from the interorbital space to the vent. Lateral with many small blackish dots, ventral immaculate except for a dark-grey M-shaped band across the gular area (Figure 4B). Transverse black bands on the dorsal surfaces of thigh, tibia, and tarsus were easily discernable.
Variation.Variation in meristic and morphometric characters among the type series are shown in Table 3. Individuals of the type series are generally similar in appearance, but show certain variation in coloration and dorsal pattern. Among the studied types, we found variation in the degree of development of mid-dorsal vertebral light line. Paratypes resemble the holotype in all aspects of morphology except for KIZ024053, KIZ024054 and KIZ024100, which do not have the thin whitish mid-dorsal stripe from tip of between eyes to vent (27%). In KIZ024098, the mid-dorsal line is broken in the scapular area. Variation in dorsal coloration is also observed: the head might be light dark green to dark brown and the dorsum is greyish brown with many large black spots.
Advertisement call.Calls were recorded at an air temperature of 26.0°C. This species had both short and long calls, though the latter were not always emitted (Figure 2). The shorter call consisted of a series of pulsed notes. Each of these notes lasted 3.0±0.4 s and was composed of 9-12 pulses/call (average 11.2±1.8). The note interval was 1.81±0.598 s, the dominant frequency was 2.0±0.03 kHz, and the second harmonic was about 3 857±0.036 kHz. The call had a slight frequency modulation.
Etymology.The specific epithet chiangmaiensis is a Latinized adjective derived from the name of Chiang Mai Province, Thailand. We suggest the common English name“Chiang Mai Rain-Pool Frog” and vernacular name in Thai “Kob-Nonglek Chiang Mai”, taken from “Kob” for frog, “Nonglek” for small swamp, “Chiang Mai” for Chiang Mai Province, Thailand.
Distribution and habitat.Fejervarya chiangmaiensissp. nov.is, to date, known only from a single locality, encompassing a lowland farm in Ban Monjong, Omkoi District, Chiang Mai, northern Thailand (N17°28'16.93", E98°27'28.26"; 460 m a.s.l.). The frogs were found calling on clods of dirt in rice fields at night during rainfall; it appears that the species inhabits disturbed habitats, including agricultural areas. Females of the new species remain unknown. The new species was found in sympatry with F. limnocharis, Occidozyga lima, and Hoplobatrachus rugulosus.
Comparisons with other congeners.
Fejervarya chiangmaiensissp. nov.can be distinguished from large- and medium-sized members of Fejervarya in external morphology, coloration, and acoustics. Fejervarya chiangmaiensissp. nov.can be distinguished from members of the sister matriline, comprised of F. granosa (central Western Ghats, India), F. pierrei (Nepal, Bangladesh and E India), and F. syhadrensis (India, Pakistan, Bangladesh, Sri Lanka, and Nepal). The SVL of male F. chiangmaiensissp. nov.(26.3-29.1 mm) overlaps with male F. granosa (29.1 mm; Kuramoto et al., 2007), F. pierrei (24.7-41.2 mm; Dubois, 1975), and F. syhadrensis (22-36 mm; Howlader, 2011b). However, the new species clearly differs in the dominant frequency of its advertisement call, which is higher (2.0 kHz) than that in F. granosa (1.7 kHz), but much lower than that in F. pierrei and F. syhadrensis (4.2 kHz and 2.7-4.1 kHz) (Kuramoto et al., 2007; Purkayastha & Matsui, 2012). Moreover, the new species can be further distinguished from F. granosa and F. pierrei by dorsum shagreened with rare low dorsal ridges and distinct glandular warts in the posterior part of the dorsum, and by moderately stout body habitus (vs. dermal ridges on the back well-pronounced, generally short or rounded and body shape relatively thick in F. granosa, Kuramoto et al., 2007; and vs. dorsum highly tuberculated, habitus stocky in F. pierrei, Dubois, 1975). The new species can be further distinguished from F. pierrei by relative finger lengths, with the second finger being shorter than the fourth finger (II<IV<I<III in F. chiangmaiensissp. nov.vs. II=IV<I<III in F. pierrei; Howlader, 2011b). Fejervarya chiangmaiensissp. nov.can be further differentiated from F. syhadrensis by head width less than head length, HW/HL rate 0.9 (vs. head broader than long, HW/HL rate 1.0 in F. syhadrensis, see Kuramoto et al., 2007) and byrelative finger lengths (II<IV<I<III in F. chiangmaiensissp. nov.vs. I=II<IV<III in F. syhadrensis; Howlader, 2011b).
The body size of male F. chiangmaiensissp. nov.differs from that of other species of Fejervarya. The new species (males 26.3-29.1 mm; Thailand) is smaller than F. sengupti (males 33.4-35.7 mm; Maghalaya, India; Purkayastha & Matsui, 2012) and much smaller than F. kudremukhensis (males 40.8-43.3 mm; Western Ghats, India; Kuramoto et al., 2007). The new species also clearly differs by the dominant frequency of the advertisement call, which is lower than that in both F. sengupti and F. kudremukhensis (2.0 kHz vs. 3.3 kHz and 3.6 kHz, respectively; as reported by Kuramoto et al., 2007; Purkyastha & Matsui, 2012). Fejervarya chiangmaiensissp. nov.can be further diagnosed from F. sengupti and F. kudremukhensis by dorsum shagreened with granules in the posterior part, and by head width less than head length, HW/HL rate 0.89 (vs. dorsum densely granulated with dermal transversal folds, and head notably wider than long, HW/HL rate 1.2 in F. sengupti, see Purkyastha & Matsui 2012; and vs. dorsum with a few short dermal ridges and interrupted reversed V-shaped ridge in the scapular area, and head wider than long, HW/HL rate 1.1 in F. kudremukhensis, data for males, see Kuramoto et al., 2007).
Although comparative data are limited, the SVL of F. chiangmaiensissp. nov.overlaps with the SVL of male F. nepalensis (23.0-37.8 mm, Nepal, NE India, Bhutan, Bangladesh; see Dubois, 1975). However, the new species clearly differs from the latter by relative finger length: II<IV<I<III and shagreened dorsum vs. II<I<IV<III and warted dorsum in F. nepalensis.
The following species of Fejervarya have greater male SVL values than that of F. chiangmaiensis sp. nov: F. mysorensis from India (37.0 mm: Dutta, 1997 as Limnonectes), F. teraiensis from Nepal (40.1-50.5 mm; Matsui et al., 2007), and F. murthii (35.0 mm: Dutta, 1997 as Limnonectes) and F. nilagirica (34.7-42.2 mm), two endemic species from India.
Although F. asmati from Bangladesh, F. keralensis from India, and F. brevipalmata from India overlap with the new species in body size (SVL 29.1-33.4 mm: Howlader, 2011a; 21.2-47.0 mm: Dutta, 1997, and 28.3-59.8 mm: Dutta, 1997 as Limnonectes, respectively), the presence of these species in northern Thailand appears to be highly improbable (see Bauer et al., 1995; Boulenger, 1905; Choudhury et al., 2001; Howlader, 2011b; Peters, 1871; Purkayastha & Matsui, 2012). The new species can be further differentiated from F. asmati by snout pointed in lateral view (vs. snout almost rounded in lateral view), by skin on dorsum shagreened without dermal folds in shape of inversed V (vs. skin on dorsum with transverse elongated ridges forming inversed V-pattern), by coloration of vocal sacs in breeding males forming a dark M-shaped pattern on the gular area (vs. characteristic butterfly-shaped spot on throat in males), and by the dominant frequency of the advertisement call, which is lower than that in F. asmati (2.0 kHz vs. 4.1-5.1 kHz) (Howlader, 2011b). The new species can be further distinguished from F. keralensis by having a comparatively larger head (HL/SVL 0.4 in F. chiangmaiensis sp. nov vs. 0.4 in F. keralensis males) and larger eyes (EL/SVL 0.1 in F. chiangmaiensis sp. nov vs. 0.1 in F. keralensis, data for males, see Kuramoto et al., 2007) and less developed small webbing; web formula: I 1-2 II 1-2? III 2-3 IV 3-1 V (vs. wide almost complete webbing in F. keralensis; web formula: I 1-2 II 2-1 III 1-1 IV 1-1 V, Kuramoto et al., 2007).
The three other species of Fejervarya from the Western Ghats in southern India, F. nilagirica, F. caperata, and F. mudduraja, can be also differentiated from the new species on the basis of body size and proportions and by presence of warts and dermal ridges on the dorsum (Kuramoto et al., 2007). Fejervarya nilagirica is a large-bodied species and can be easily distinguished from the small-bodied F. chiangmaiensissp. nov.(male SVL 26.3-29.1); and further distinguished by the numerous warts and dermal ridges on the dorsum (vs. smooth to shagreened dorsum with glandular warts in posterior part in F. chiangmaiensissp. nov.) and by relatively smaller eyes, EL/SVL min 0.1-0.2 in the new species. Although F. caperata overlaps with F. chiangmaiensissp. nov.in SVL (mean SVL being 33 mm in females and 29 mm in males), it can be distinguished by its relatively slender body habitus (vs. moderately stout body habitus in the new species), by long dermal ridges on the dorsum forming four longitudinal lines (vs. smooth to shagreened dorsum with glandular warts in posterior part in F. chiangmaiensissp. nov.), and by relative finger lengths IV<II<I<III (vs. relative finger lengths II<IV<I<III in the new species). Fejervarya mudduraja can be distinguished by its large body size, with mean SVL of females being 45 mm (no information on male SVL available) (vs. small body size in F. chiangmaiensissp. nov., SVL 26.3-29.1), presence of long dermal ridges on the back arranged into four longitudinal lines (vs. smooth to shagreened dorsum with glandular warts in posterior part in M. chiangmaiensissp. nov.), and by head wider than long, HW/HL=1.1 (vs. head width less than head length, HW/HL=0.9 in F. chiangmaiensissp. nov.).
Fejervarya murthii (Tamil Nadu, Karnataka, south India) can be distinguished by the presence of two triangular patches bearing pearl-like papillae on the breast in males, and presence of the papillae in the anterior part of the lower jaw (Pillai, 1979) (vs. no such discernible papillae in F. chiangmaiensissp. nov.).
The new species can be easily diagnosed from F. teraiensis inhabiting Nepal and NE India by its smaller body size (SVL 26.3-29.1 vs. SVL 37.8-44.1 in males of F. teraiensis; Howlader, 2011b), relative finger lengths (II<IV<I<III in F. chiangmaiensissp. nov.vs. II=IV<I<III in F. teraiensis; Howlader, 2011b) and head width less than head length, HW/HL rate 0.9 (vs. head width almost equal to head length, HW/HL rate 1.0 in F. teraiensis).
Fejervarya rufescens from southern India can be distinguished by having stocky body habitus (vs. moderately stout habitus in F. chiangmaiensissp. nov.), highly tuberculated skin on the dorsum with pronounced transverse dermal ridges forming an inverse V-pattern (vs. shagreened dorsum with rare low dorsal ridges in in F. chiangmaiensissp. nov.), and reddish coloration on the dorsum in breeding males (vs. grayish or greenish dorsal coloration in breeding males of F. chiangmaiensissp. nov.). Sri Lankan F. kirtisinghei and F. greenii, can be easily differentiated by the dorsum covered with well-developed long continuous dermal ridges (vs. shagreeneddorsum with rare low dorsal ridges, never forming continuous rows in F. chiangmaiensissp. nov.).
Comparison of F. chiangmaiensissp. nov.with certain dicroglossid species reported for the region is complicated due to their unclear taxonomic status. Locality of F. brevipalmata, originally designated as “Pegu, Myanmar”, appears to be uncertain. This species likely occurs in the Western Ghats of southern India. Its taxonomic status is unclear (AmphibiaWeb, 2016; Boulenger, 1920). Furthermore, F. sauriceps and F. parambikulamana, two endemic species from Kerala and Karnataka of southern India, are known only from holotypes that appear to be lost. Fejervarya sauriceps is supposed to differ from other known Fejervarya by a very small tongue, unique triangular pit on the snout, a brown venter, and wide interorbital width (more than twice the upper eyelid width), whereas F. parambikulamana is diagnosed by smooth dorsum and comparatively longer legs (Kuramoto et al., 2007); both differ from F. chiangmaiensissp. nov.Taxonomic validity and systematic status of these species requires further investigation (AmphibiaWeb, 2016; Frost, 2016) and some researchers doubt their validity (Matsui et al., 2007; Purkayastha & Matsui, 2012). Similar concerns have been raised on some other dicroglossid frog species known in regions nearby, in particular F. altilabris (Blyth) from Myanmar, F. frithii (Theobald) from Bangladesh, and F. assimilis (Blyth) and F. brama (Lesson) from India (Matsui et al., 2007). Fejervarya chiangmaiensissp. nov.can be further differentiated by its smaller body size from F. orissaensis (Orissa, India; Dutta, 1997) (males 26.3-29.1 mm vs. 36.2-47.2 mm in F. orissaensis Dutta (1997) as Limnonectes). Furthermore, F. chiangmaiensissp. nov.can be distinguished from the two other miniaturized species of Fejervarya (F. sahyadris and F. chilapata) by a larger SVL in males (male of F. chiangmaiensissp. nov., mean SVL=28.1±1.0; n=12 vs. males of F. sahyadris and F. chilapata, mean SVL=18.4±6.0 mm; n=10, SVL=20.0±7.0 mm; n=8, respectively), by lacking a white stripe on the upper lip (vs. white stripe present in F. sahyadris and F. chilapata), by lacking light dorsolateral lines (vs. light dorsolateral lines present in F. sahyadris and F. chilapata), by having shagreened dorsal and lateral surfaces of head and body including body flanks, posterior part of dorsum with distinct, round glandular warts, continuing on dorsal surfaces of legs and arms (vs. dorsal and lateral parts of head and body smooth; posterior part of back with indistinct, glandular warts in F. sahyadris and F. chilapata), and by different advertisement call (F. sahyadris (3.6-4.4 kHz) and F. chilapata (2.0 kHz) have a higher dominant frequencies than that of F. chiangmaiensissp. nov.(2.0 kHz)).
Numerous differences in morphology, coloration, acoustics, and mtDNA gene sequences give support to recognizing the specimens collected in Chiang Mai Province of Thailand as a new species. Accordingly, description is necessary to accurately document the anuran biodiversity of Thailand.
ACKNOWLEDGEMENTS
We thank the local Forestry Department and National Reserve for assistance in the field in Thailand. The Institute of Animals for Scientific Purpose Development (IAD) provided permission (No. U1-01205-2558) in Thailand. We gratefully acknowledge Parinya Pawangkhanant and Michael Cota for assistance in Thailand and providing literature.
REFERENCES
AmphibiaWeb. 2016[2016-06-30]. Information on amphibian biology and conservation. Berkeley, California: AmphibiaWeb, http://amphibiaweb.org/. Annandale N. 1919. The fauna of certain small streams in the Bombay Presidency. v. Notes on freshwater fish mostly from the Satara and Poona districts. Records of the Indian Museum,16(1): 125-138.
Bauer AM, Günther R, Klipfel M. 1995. Synopsis of taxa. In: Bauer AM, Günther R, Klipfel M. Herpetological Contributions of W.C.H. Peters (1815-1883). Oxford: Society for the Study of Amphibians and Reptiles, 39-81.
Boulenger GA. 1905. Description of a frog from Ceylon, hitherto confounded with Rana limnocharis. Spolia Zeylanica,2: 73-74.
Boulenger GA. 1920. A monograph of the South Asian, Papuan, Melanesian and Australian frogs of the genus Rana. Records of the Indian Museum,20: 1-226.
Choudhury NK, Hussain BK, Baruah M, Saikia S, Sengupta S. 2001. Amphibian fauna of Kamrup District, Assam, with notes on their natural history. Hamadryad,26(2): 276-282.
Dinesh KP, Vijayakumar SP, Channakeshavamurthy BH, Toreskar VR, Kulkarni NU, Shanker K. 2015. Systematic status of Fejervarya (Amphibia, Anura, Dicroglossidae) from South and SE Asia with the description of a new species from the Western Ghats of Peninsular India. Zootaxa,3999(1): 79-94.
Djong HT, Matsui M, Kuramoto M, Nishioka M, Sumida M. 2011. A new species of the Fejervarya limnocharis Complex from Japan (Anura, Dicroglossidae). Zoological Science,28(12): 922-929.
Dubois A. 1975. Un nouveau complexe d’especes jumelles distinguées par le chant: les grenouilles du Nepal voisines de Rana limnocharis Boie (Amphibiens, Anoures). Comptes Rendus de l’Académie des Sciences, Série D,281: 1717-1720.
Dubois A. 1984. Note preliminaire sur le groupe de Rana limnocharis Gravenhorst, 1829 (Amphibiens, Anoures). Alytes,3(4): 143-159.
Dubois A, Ohler A, Biju SD. 2001. A new genus and species of Ranidae (Amphibia, Anura) from south-western India. Alytes,19(2-4): 53-79.
Dutta SK. 1997. A new species of Limnonectes (Anura: Ranidae) from Orissa, India. Hamadryad,22(1): 1-8.
Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32: 1792-1797.
Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution,39(4): 783-791.
Frost DR. 2016(2016-03-23). Amphibian species of the World 6.0, an online reference. New York, USA: American Museum of Natural History, http:// research.amnh.org/herpetology/amphibia/index.html.
Gravenhorst JLC. 1829. Deliciae Musei Zoologici Vratislaviensis. Fasciculus primus.Chelonios et Batrachia. Leipzig: Leopold Voss.
Günther ACLG. 1859. Catalogue of the Batrachia Salientia in the Collection of the British Museum. London: Taylor and Francis.
Günther ACLG. 1869. First account of species of tailless batrachians added to the collection of the British Museum. Proceedings of the Zoological Society of London,1868: 478-490.
Hallowell E. 1861 "1860". Report upon the Reptilia of the North Pacific
Exploring Expedition, under command of Capt. John Rogers, U.S.N. Proceedings of the Academy of Natural Sciences of Philadelphia,12: 480-510.
Hasan M, Islam MM, Khan MR, Igawa T, Alam MS, Djong HT, Kurniawan N, Joshy H, Sen YH, Belabut DM, Kurabayashi A, Kuramoto M, Sumida M. 2014. Genetic divergences of South and Southeast Asian frogs: a case study of several taxa based on 16S ribosomal RNA gene data with notes on the generic name Fejervarya. Turkish Journal of Zoology,38: 389-411.
Howlader MSA. 2011a. A new species of Fejervarya (Anura: Dicroglossidae) from Bangladesh. Zootaxa,2761: 41-50.
Howlader MSA. 2011b. Cricket frog (Amphibia: Anura: Dicroglossidae): two regions of Asia are corresponding two groups. Bonnoprani-Bangladesh Wildlife Bulletin,5(1-2): 1-7.
Huelsenbeck JP, Hillis DM. 1993. Success of phylogenetic methods in the four-taxon case. Systematic Biology,42(3): 247-264.
Inger RF. 1966. The systematics and zoogeography of the Amphibia of Borneo. Fieldiana Zoology,52: 1-402.
Jerdon TC. 1853. Catalogue of reptiles inhabiting the Peninsula of India. Journal of the Asiatic Society of Bengal,22: 522-534.
Kocher TD, Thomas WK, Meyer A, Edwards SV, P??bo S, Villablanca FX, Wilson AC. 1989. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proceedings of the National Academy of Sciences of the United States of America,86(16): 6192-6200.
Kosuch J, Vences M, Dubois A, Ohler A, B?hme W. 2001. Out of Asia: mitochondrial DNA evidence for an Oriental origin of tiger frogs, genus Hoplobatrachus. Molecular Phylogenetics and Evolution,21(3): 398-407.
Kotaki M, Kurabayashi A, Matsui M, Kuramoto M, Djong HT, Sumida M. 2010. Molecular phylogeny for the diversified frogs of genus Fejervarya (Anura: Dicroglossidae). Zoological Science,27(5): 386-395.
Kotaki M, Kurabayashi A, Matsui M, Khonsue W, Djong HT, Tandon M, Sumida M. 2008. Genetic divergences and phylogenetic relationships among the Fejervarya limnocharis complex in Thailand and neighboring countries revealed by mitochondrial and nuclear genes. Zoological Science,25(4): 381-390.
Kurabayashi A, Kuramoto M, Joshy H, Sumida M. 2005. Molecular phylogeny of the ranid frogs from Southwest India based on the mitochondrial ribosomal RNA gene sequences. Zoological Science,22(5): 525-534.
Kuramoto M, Joshy SH, Kurabayashi A, Sumida M. 2007. The genus Fejervarya (Anura: Ranidae) in central Western Ghats, India, with description of four new cryptic species. Current Herpetology,26(2): 81-105. Manamendra-Arachchi K, Gabadage D. 1996. Limnonectes kirtisinghei, a new species of ranid frog from Sri Lanka. Journal of South Asian Natural History,2(1): 31-42.
Manthey U, Grossmann W. 1997. Amphibien & Reptilien Südostasiens. Münster: Natur und Tier Verlag, 512.
Matsui M. 1984. Morphometric variation analyses and revision of the Japanese toads (Genus Bufo, Bufonidae). Contributions from the Biological Laboratory, Kyoto University,26(3-4): 209-428.
Matsui M, Toda M, Ota H. 2007. A new species of frog allied to Fejervarya limnocharis from the southern Ryukyus, Japan (Amphibia: Ranidae). Current Herpetology,26(2): 65-79.
Meegaskumbura M, Bossuyt F, Pethiyagoda R, Manamendra-Arachchi K, Bahir M, Milinkovitch MC, Schneider CJ. 2002. Sri Lanka: an amphibian hot spot. Science,298(5592): 379.
Nylander JAA. 2004. MrModeltest V2. Program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University.
Ohler A, Deuti K, Grosjean S, Paul S, Ayyaswamy AK, Ahmed MF, Dutta SK. 2009. Small-sized dicroglossids from India, with the description of a new species from West Bengal, India. Zootaxa,2209: 43-56.
Orlov NI, Ananjeva NB. 2007. Amphibians of South-East Asia. Proceedings of the Zoological Institute RAS,309: 1-269.
Peters WCH. 1871. über einige Arten der herpetologischen Sammlung des Berliner zoologischen Museums. Monatsberichte der K?niglichen Preussische Akademie des Wissenschaften zu Berlin,1871: 644-652.
Pillai RS. 1979. A new species of Rana (family Ranidae) from Western Ghats, S. India. Bulletin-Zoological Survey of India,2: 39-42.
Purkayastha J, Matsui M. 2012. A new species of Fejervarya (Anura: Dicroglossidae) from Mawphlang, northeastern India. Asian Herpetological Research,3(1): 31-37.
Rao CRN. 1922. Notes on Batrachia. Journal of the Bombay Natural History Society,28: 439-447.
Rao CRN. 1937. On some new forms of Batrachia from S. India. Proceedings of the Indian Academy of Sciences,6(6): 387-427.
Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics,19(12): 1572-1574.
Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular Cloning: A Laboratory Manual. 2nded. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory Press, 1659.
Savage JM. 1975. Systematics and distribution of the Mexican and Central American stream frogs related to Eleutherodactylus rugulosus. Copeia,1975(2): 254-306.
Smith MA. 1930. The reptilia and amphibia of the malay peninsula. Bulletin of the Raffles Museum,3: I-XVIII+1-149. Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology,57(5): 758-771.
Stoliczka F. 1870. Observations on some Indian and Malayan Amphibia and Reptilia. Journal of the Asiatic Society of Bengal,39: 134-157.
Stuart BL, Chuaynkern Y, Chan-Ard T, Inger RF. 2006. Three new species of frogs and a new tadpole from eastern Thailand. Fieldiana Zoology,111: 1-19. Swofford DL. 2003. PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods), version 4.0b10. Sunderland, MA: Sinauer Associates.
Taylor EH. 1920. Philippine Amphibia. Philippine Journal of Science,16: 213-359.
Taylor EH. 1962. The amphibian fauna of Thailand. The University of Kansas Science Bulletin,43: 265-599.
Vences M, Thomas M, Bonett RM, Vieites DR. 2005a. Deciphering amphibian diversity through DNA barcoding: chances and challenges. Philosophical Transactions of the Royal Society B: Biological Sciences,360(1462): 1859-1868.
Vences M, Thomas M, van der Meijden A, Chiari Y, Vieites DR. 2005b. Comparative performance of the 16S rRNA gene in DNA barcoding of amphibians. Frontiers in Zoology,2: 5.
Received: 20 July 2016; Accepted: 17 October 2016
Foundation items: This work was supported by the Program of the Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences (Y4ZK111B01: 2015CASEABRI002), National Natural Science Foundation of China (31501843), and Animal Branch of the Germplasm Bank of Wild Species, Chinese Academy of Sciences (Large Research Infrastructure Funding). Chatmongkon Suwannapoom was sponsored by the Chinese Academy of Sciences Visiting Fellowship for Researchers (Postdoc.) from Developing Countries (2013FFS130015). Nikolay A. Poyarkov was supported by the Russian Science Foundation (RSF grant No.14-50-00029). Robert Murphy was supported by a NSERC Discovery Grant (3148), the ROM Foundation, and the ROM Members Volunteer Committee
*Corresponding author, E-mail: chej@mail.kiz.ac.cn
10.13918/j.issn.2095-8137.2016.6.327
- Zoological Research的其它文章
- Re-evaluating data quality of dog mitochondrial, Y chromosomal, and autosomal SNPs genotyped by SNP array
- Molecular characterization and functional analysis of a piscidin gene in large yellow croaker (Larimichthys crocea)
- Patterns of change in the population and spatial distribution of oriental white storks (Ciconia boyciana) wintering in Poyang Lake
- The geographical distribution of grey wolves (Canis lupus) in China: a systematic review
- Acknowledgments to reviewers of Zoological Research
- A clarification of authorship in an old publication

