Preliminary Study on Tissue Culture Technique of Brassica campestris L.ssp.chinensis(L.)Makino var.utilis
Yanchun QIAO, Hongdi HUANG, Hua ZHANG, Guangguang LI, Yansong ZHENG, Zizhu LIU
Guangzhou Academy of Agricultural Sciences, Guangzhou 510308, China
Brassica campestris L.ssp. chinensis var. utilis is one of the specialty vegetables in South China. It has been cultivated in Guangdong and Guangxi for a long time. The variety resources were very abundant[1]. Currently, conventionality breeding is mainly adopted in the breeding of Brassica campestris L.ssp. chinensis var. utilis. Because the period of conventionality breeding is long and the efficiency is low, and conventionality breeding is affected by apparent growth status, elite varieties are hardly to be bred.Moreover,in the aspect of disease resistance breeding,the weakness of conventionality breeding is more obvious. Because disease resistance characters are hardly to be maintained in progenies, the relative index parameters of disease resistance are hardly to be confirmed.Haploid plants of more than 50 kinds of plants have been obtained by haploid breeding since which was applied in 1970s[2]. Regeneration plants of multiple Cruciferae crops were obtained through anther culture or microspore culture.The Cruciferae crops included Brassica campestris[3], B. oleracea var. botrytis[4], B. oleracea[5],B. chinensis[6], Raphanus sativus[7],B. rapa ssp.chinensis[8],B.compestris var. purpurea[9], B. alboglabra[10], etc.Currently, there are several reports about the anther culture technique of Brassica campestris L. ssp. chinensis var. utilis but the techniques are not mature and most researches are still stayed at the embryoid phase[11-14].The tissue culture operations vary from person to person and the key tech-niques cannot be copied. This experiment tried to confirm the best period and statue of anther for culture according to the morphology of pollen and anther of Brassica campestris L. ssp.chinensis var. utilis. According to tissue culture, operable technical condition and method of anther tissue culture were quested and scientific basis was also provided for elite variety breeding of Brassica campestris L.ssp.chinensis var.utilis.
Materials and Methods
Materials
Testing materials were taken from Flower base of Guangzhou Academy of Agricultural Science. Thirty two germplasm resources of Brassica campestris L. ssp. chinensis var. utilis were used in the test. Six varieties including 49-19 caixin, Youlv 50 tian,lvbao 70 tian, Youlv 702, Youlv 801 and Chixin caixin 4 were bred by Guangzhou Academy of Agricultural Science. The other 26 materials included four early maturing varieties,six middle maturing varieties, four late maturing varieties, six wide leaf varieties and six gracile leaf varieties. The phenotypes of the testing materials had significant differences, which provided abundant genotypic materials.
Measurement of pollen viability
Pollen viability of Brassica campestris L. ssp. chinensis var. utilis was measured based on the medium germination method of Liu et al[15]. The culture medium was 100 mg/L sucrose+30 mg/L boric acid.
Anther culture
Callus induction Materials with different genotypes were cultured on different media with different levels of growth regulators for callus induction.The basic medium was MS with the addition of 3% sugar, 6 g/L agar and 8% coconut milk. KT and 2, 4-D were used as growth regulators. The dosage of growth regulators were screened through simple factor design of experiment. The dosages of KT were set as 0.5,1.0 and 2.0 mg/L,and the dosages of 2, 4-D were set as 1.0 and 2.0 mg/L.
Differentiation culture of bud Tight calluses were inoculated on culture media for the differentiation of adventitious bud. MS+ 2% sucrose+ 6 g/L agar was used as the basic culture medium. Additives and the combination of growth regulators were screened by double-factor design(Table 1).Thirty calluses were cultured on each medium.

Table 1 Selection of bud differentiation medium for Brassica campestris ssp.chinensis var.utilis
Tissue culture of cotyledon-cotyledonary petioles
Preparation of materials and induction of callus Seeds of Brassica campestris L. ssp. chinensis var. utilis(49 -19 Caixin) were sterilized with 70% ethyl alcohol for 30 s and 0.1%mercury bichloride for 6-8 min, and cultured on MS media under dark for about 15 d. The cotyledon-cotyle-donary petioles were selected as explants and cultured on media for callus induction. The culture media were MS+1.0 mg/L KT + 1.0 mg/L 2, 4-D +3% sugar + 6 g/L agar + 8% coconut milk. Three explants were inoculated in each bottle and the experiment was repeated three times. Induction rates of calluses were counted after 15 d dark culture.
Induction of adventitious buds
Cotyledon-cotyledonary petiole callus were inoculated on induction media for adventitious buds. The media were MS+2.0 mg/L 6-BA+0.5 mg/L NAA+1.0 g/L activated carbon+2%sugar +6 g/L agar or MS + 2.0 mg/L ZT + 0.5 mg/L IAA + 0.5 g/L AgNO3+ 1.0 g/L activated carbon + 2% sugar + 6 g/L agar. Each treatment were inoculated for ten bottles and repeated three times. Induction ratios of adventitious buds were counted after 15 d light culture.
Rooting culture The induced adventitious buds were transformed on rooting culture media (1/4 MS or 1/8 MS+0.1 mg/L IBA+2%sugar+6 g/L agar). The induced adventitious buds were inoculated for ten bottles with two plants each bottle and three replicates.Rooting situation was observed after 15 d light culture.
Results and Analysis
Pollen morphology of Brassica campestris L. ssp. chinensis var.utilis at mid-late uninucleate stage
For anther and pollen culture,development stage of pollen is very important for the improvement of plant regeneration rate. Identifying the development stage of pollen accurately and inoculating pollens appropriately are key operation techniques of anther and pollen culture.Development stage of pollens was observed and confirmed with microscope. Pollens at mid-late uninucleate stage were mainly selected for anther culture. At the mid-late uninucleate stage, flowers were not bloom and flower stigmas were slightly higher than petals, and most pollen cells were haploid or diploid and polyploidy doubled naturally(Fig.1).
Pollen viability
Pollen viability was measured with germination method to determine the stage of strong viability. It could be seen from Table 2 that the samples were harvested on September 7 and November 15, 2012, respectively, and the germination rates of Brassica campestris L. ssp. chinensis var. utilis were at a middle level, which illustrated that pollen viability of the tested materials were general. The germination rate of pollens harvested on September 7,2012 was slightly higher than 50% after germinated for 4 h while the germination rate of pollens harvested on November 15, 2012 could reach 70%,which illustrated that at the most suitable growth stage of Brassica campestris L. ssp. chinensis var. utilis, November, pollen viability was relatively stronger and pollens were more suitable for pollen culture.Elliptical pollens were closely dotted on the inoculated culture media (Fig.2 A). Small protuberances occurred on the edge of the elliptical pollens after which were cultured at 25 ℃for 2 h(Fig.2B). The small protuberances elongated gradually after the pollens cultured for 4 h and types of the pollens looked like small tadpoles (Fig.2C), thus this stage was the most suitable stage for the statistics of pollen germination condition.The germinated long pollen tubes intertwined together after cultured for 8 h, which was not conducive for the statistics of pollen germination rate.
Bud differentiation and induction of anther callus
Induction rate of anther callus was observed and counted. The results showed that calluses induced on MS+1.0 mg/L KT + 1.0 mg/L 2, 4-D + 3%sugar + 6 g/L agar + 8% coconut milk were tightness that the culture medium was suitable for callus induction.MS+2.0 mg/L 6-BA + 0.5 mg/L NAA + 1.0 g/L activated carbon+2%sugar+6 g/L agar or MS + 2.0 mg/L ZT + 0.5 mg/L IAA+0.5 g/L AgNO3+1.0 g/L activated carbon + 2% sugar + 6 g/L agar were suitable for the induction of adventitious buds(Table 1,differentiation rate was 36.7%). During the culture process,calluses induced with anthers of Brassica campestris L.ssp.chinensis var. utilis were found to be easily differentiated and there were many trichomes appeared (Fig.3). After the tight calluses were transformed to differential media, calluses turned green and there were many small buds generated but only several bud points could differentiate young buds (Fig.4).Browning phenomenon became severer with the growth of young buds.The browning condition cannot be improved though Vc was added to the culture media.The phenomenon might be resulted from autotoxicity of the materials that the young buds cannot be further differentiated and then they browned to death.
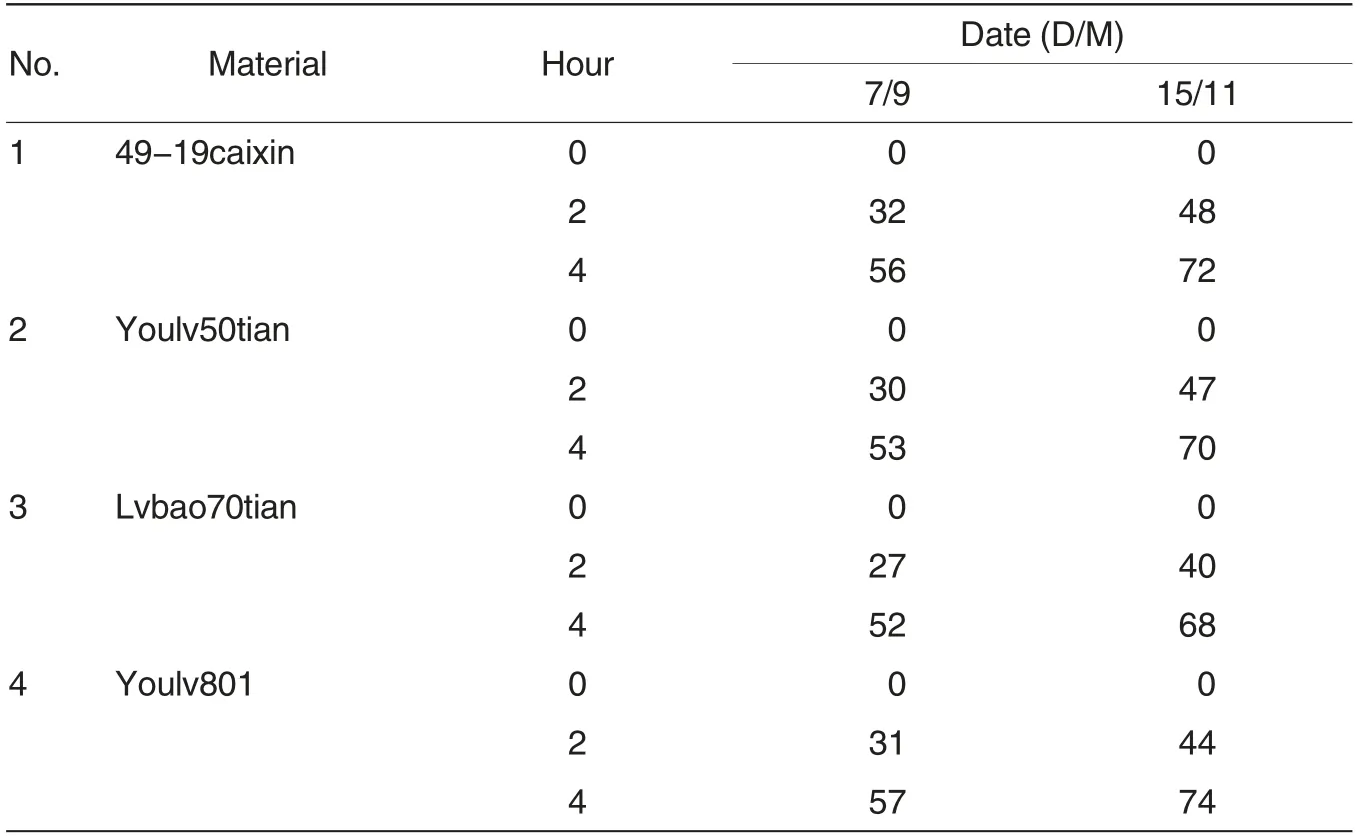
Table 2 Pollen germination rate(%)of Brassica campestris ssp.chinensis var.utilis
Explant culture and establishment of regeneration system
The differentiation rates of buds generated from calluses through anther induction were low and the differ-entiation qualities were bad. Cotyledon-cotyledonary petioles were used as explants in this study for tissue culture and plant regeneration, thus to provide ideas for the improvement of bud differentiation rate by anther culture.The results showed that most explant tissues directly differentiated into adventitious buds without callus stage(Fig.5A, B) when cotyledon-cotyledonary petioles of Brassica campestris L. ssp. chinensis var. utilis were used for tissue culture.The induction rate of adventitious buds reached 85% and the speed of bud multiplication was fast. The well developing adventitious buds were transformed to rooting media, 1/4MS or 1/8MS +0.1 mg/L IBA +2% sugar + 6 g/L agar, thus to obtain intact plants (Fig.5C) and the rooting rate reached 80%. It could be seen that, using cotyledon-cotyledonary petioles as explants to directly differentiate adventitious buds might be a characteristic of Brassica campestris L. ssp. chinensis var. utilis whereas obtaining regeneration plants through calluses obtained from anther culture was had. The future research thought should be further adjusted.
Discussions
Inbreeding of more generation needed in conventional cross-breeding due to the segregation of genetic characters is leaved out when anther and pollen culture were adopted to breed haploid or homozygous diploid plants.Moreover, the haploid or homozygous diploid plants can be used as inbred lines for the utilization of heterosis and the breeding cycle is greatly shortened that it is always a research emphasis of breeding workers[16]. Anther culture and pollen culture of Brassicaceous vegetables has been deeply studied,such as Chinese cabbage, B. pekinensis, B. oleracea var. gemmifera,B. oleracea var. capitata, B. oleracea var. botrytis, broccoli, B. juncea and B. rapa, and some studies has been applied in breeding practice[17].Though anther culture and pollen culture of Brassica campestris L. ssp. chinensis var. utilis has been reported, the technology is still immature.Fujian Institute of Agricultural Sciences once studied the technology of pollen culture of Brassica campestris L. ssp. chinensis var.utilis but there was no report about it. Xiao et al. observed the microsporogenesis and male gametophyte development of Brassica campestris L.ssp. chinensis var. utilis[18]. Professor Feng in Shenyang Agricultural University studied the techniques for microspore culture of most Brassicaceous vegetables, but the techniques were immature and needed to be further studied[12].
The results in this study indicated that not bloomed flower bud and higher flower stigmas than petals were the morphological characteristics of Brassica campestris L. ssp. chinensis var.utilis pollen at mid-late uninucleate stage. The germination rates of pollens in autumn and winter were higher than that in summer.The culture medium for callus induction of Brassica campestris L. ssp. chinensis var. utilis anther was MS + 1.0 mg/L KT + 1.0 mg/L 2,4-D+3%sugar+6 g/L agar+8% coconut milk. Differentiation media of adventitious bud were MS + 2.0 mg/L 6-BA + 0.5 mg/L NAA + 1.0g/L activated carbon + 2% sugar + 6 g/L agar or MS + 2.0 mg/L ZT + 0.5 mg/L IAA+0.5 g/L AgNO3+1.0 g/L activated carbon + 2% sugar + 6 g/L agar.The induction rate of adventitious buds obtained from anther culture was 36.7% and the induction rate was not improved when exogenous BR and TDZ were added. The regeneration rate of plants obtained with cotyledoncotyledonary petioles as explants reached 80%.The rooting media were 1/4 MS or 1/8MS+0.1 mg/L IBA+2%sugar+6 g/L agar.In the research,intact regeneration plants were obtained by cotyledon-cotyledonary petiole culture but not anther culture. Adventitious buds were directly differentiated without callus stage, which was a characteristic of the tissue culture of Brassica campestris L. ssp. chinensis var.utilis[19].It can be seen that to obtain diploid plants of Brassica campestris L. ssp. chinensis var. utilis through anther culture is difficult.
Anther culture is not only affected by culture medium and growth regulatory substances but also affected by environment factors.The growth environment of donor plants is critical for anther and pollen culture, especially temperature and light. Zhang[20]conducted microspore culture of Chinese cabbage at 15-20 ℃with 14-16 h/d light and found that the occurrence rate of embryoids and regeneration rate of plants were high. For anther culture of cauliflower, the anther culture effect of donor plants harvested at 10-20 ℃greenhouse in spring was the best. The materials of Brassica campestris L. ssp. chinensis var. utilis used in this study were grew in field and the most appropriate growth seasons of Brassica campestris L. ssp.chinensis var. utilis were autumn and winter. The illumination time is short during October to December that the pollen viability is not strong enough. It can be seen that anther and pollen culture are affected by the growth environment of donor plants in addition to the culture condition, culture medium and growth regulatory substance.Brassica campestris L. ssp. chinensis var. utilis may be more sensitive to light. Above all, because of the many influence factors of anther and pollen culture, and the more strict culture condition needed by Brassica campestris L.ssp.chinensis var.utilis,how to screen appropriate factors,media and growth regulatory substances for anther and pollen culture,and construct an perfect system for diploid breeding of Brassica campestris L. ssp. chinensis var. utilis needs to be further studied.
[1]LI GG (李光光), ZHANG H (張華),HUANG HD (黃紅弟), et al. Research progress on flowering Chinese cabbage breeding in Guangdong Province (廣東省菜薹(菜心)育種研究進展) [J]. China Veget(中國蔬菜),2011(20):9-14.
[2]ZHANG L (張麗), WANG J (王靜).Progress of haploid breeding in Cruciferae crops(十字花科作物單倍體育種研究進展) [J]. J Anhui Agri Sci(安徽農(nóng)業(yè)科學),2003,31(2):231-234.
[3]KELLER WA, ARMSTRONG KC. Embryogenesis and plant regeneration in Brassica napusanther cultures [J].Can J Bot,1977,55(10):1383-1388.
[4]FARHAM MW, CANIGLIA EJ, THOMAS CE. Efficient ploidy determination of anther-derived broccoli[J].Hort Science,1988,33(2):323-327.
[5]GORECKA K.Obtaining of homozygous lines of head cabbage (Brassica oleracea L. var. capitata L.) with aid of an-ther culture[J].Prace Habilitacyjne,1998(14):1-71.
[6]GENG JF(耿建峰), YUAN YX(原玉香),ZHANG XW (張曉偉),et al.A new Chinese cabbage:Yuyuan 50(利用游離小孢子培養(yǎng)技術(shù)育成抗熱大白菜新品種豫園) [J]. Acta Hort Sin(園藝學報), 2002,29(1):89.
[7]MEI SY (梅時勇),YANG BG (楊保國),YAO F, (姚芳) et al. Study on anther culture technique of the radish (蘿卜花藥培養(yǎng)實驗) [J].China Veget (中國蔬菜),2002(1):35-36.
[8]FENG H(馮輝),YANG S(楊碩),WANG CN (王超楠), et al. Obtaining and utilization of DH lines in pakchoi(Brassica rapa L.ssp.chinensis L.) (青梗菜優(yōu)異DH 系的創(chuàng)制與利用)[J].Sci Agri Sin(中國農(nóng)業(yè)科學),2009,42(9):3195-3202.
[9]WANG TT (王濤濤), LI HX (李漢霞),ZHANG JH (張繼紅),et al.Isolated microspore culture and plant regenerationin purple fl owering stalk (Brassica compestris ssp. chinensis var. pupurea Hort.)(紅菜薹游離小孢子培養(yǎng)與植株再生)[J].J Wuhan Bot Res(武漢植物學研究),2004,22(6):569-571.
[10]HE HJ (何杭軍),WANG XW (王曉武),WANG BL (汪炳良). Embryogenesis and plant regeneration of Chinese Kale via isolated microspore culture(芥藍游離小孢子培養(yǎng)初報)[J]. Acta Hort Sin(園藝學報),2004,31(2):239-240.
[11]ZHU YH(朱允華),LIU Q(劉清),WU CL(吳朝林), et al. Study on embryoid induction of Brassica parachinensis Bailey anther culture (菜薹花藥培養(yǎng)誘導胚狀體的研究)[J].Biotechn Bull (生物技術(shù)通報),2008(2):136-139.
[12]LIU RE (劉如娥).Research on the anther culture techniques for several vegetable crops in Brassica campestris(蕓薹屬蕓薹種幾種主要蔬菜作物花藥培養(yǎng)技術(shù)研究) [D]. Shenyang:Shenyang Agricultural University(沈陽:沈陽農(nóng)業(yè)大學),2008:8-65.
[13]WU YF (吳藝飛),DING ZY (丁茁荑),XIAO J (肖杰), et al. Affecting factors for embryo rate of anther culture of flowering Chinese cabbage (菜薹花藥培養(yǎng)出胚率的影響因素研究)[J]. Hunan Agri Sci (湖南農(nóng)業(yè)科學),2012(7):8-11.
[14]LIU SH (劉勝洪),ZENG MH (曾慕衡),LIANG H (梁紅),et al.Study on some factors of culture of Brassica napus CMS lines and flowering Chinese cabbage hybrid anther(甘藍型油菜不育系與菜心雜交后代花藥培養(yǎng)中若干因素的研究)[J].Crops(作物雜志),2007(3):58-60.
[15]LIU HC(劉會超),JIA WQ(賈文慶),JIA GR(賈國瑞).Studies on pollen viability and storage characteristics of Prunus ceraifera (紫葉李花粉生活力及貯藏特性研究)[J].J Anhui Agri Sci(安徽農(nóng)業(yè)科學),2010,38(9):4507-4508.
[16]GUO JY(郭金英), SHEN SX(申書興),CHEN XP (陳雪平),et al.Progress of isolated microspore cultures of Cruciferae vegetabale crops (十字花科蔬菜游離小孢子培養(yǎng)研究進展)[J].J Agri Univ Hebei(河北農(nóng)業(yè)大學學報),2002,25(S1.):122-124.
[17]WU J(武劍), GONG YQ(龔義勤), LIU LW (柳李旺),et al.Advances on plantlet induction from pollen of Cruciferae vegetables (十字花科蔬菜花粉植株誘導技術(shù)研究進展) [J].J Anhui Agri Sci(安徽農(nóng)業(yè)科學), 2000, 28 (6): 747-749,754.
[18]XIAO XF (肖旭峰), TANG J (唐杰),ZHOU QH(周慶紅).Research and development of male gametophyte of flowering Chinese cabbage microspore (菜心小孢子發(fā)生及雄配子體發(fā)育的研究)[J].Mod Gard(現(xiàn)代園藝),2013(3):7-9.
[19]CHENG YJ (程玉瑾),AI KF (艾珂飛),HUANG YQ (黃穎茜), et al. Plant regeneration of Brassica parachinensis cultured in vitro (菜心離體培養(yǎng)植株再生的研究)[J].J S China Agri Univ(Nat Sci)(華南農(nóng)業(yè)大學學報:自然科學版),2002,23(4):89.
[20]ZHANG FL(張鳳蘭), KUGINUKI Y(釘貫靖久),YOSHIKAWA H (吉川宏昭).The influence of growth conditions of donor plants on the microspore culture in Chinese cabbage (環(huán)境條件對白菜小孢子培養(yǎng)的影響)[J]. Acta Agri Boreali-Sin(華北農(nóng)學報),1994,9(1):95-100.
 Agricultural Science & Technology2015年10期
Agricultural Science & Technology2015年10期
- Agricultural Science & Technology的其它文章
- Effects of Specific Gravity-based Seed Grading on Seed Germination,Seedling Emergence and Grain Yield of Hybrid Rice
- Effects of NaCl Stress on Seed Germination of Four Canavium album Raeuseh Cultivars
- Application Effects of Ultra-fine Powder Shaped Maize Seed Coating Agent in Spring Sowing areas in northeast China
- Breeding and Application of a Japonic Rice Cytoplasmic Male Sterility Line,E-Jing A
- Effect of Low Temperature and Sparse Light Conditions on Cold Tolerance of Different Rice Lines at Seedling Stage
- Molecular Marker Assisted Selection for Fusarium Wilt Resistance Breeding in Watermelon(Citrullus lanatus)
