Establishment of Rapid Propagation Technique for Cotyledon of Solanum torvum
Yikui WANG, Wenjia LI, Yaqin JIANG, Yan LI, Yongguan WU, Dexian KANG, Yanyan FANG
1. Vegetable Research Institute of Guangxi Academy of Agricultural Sciences, Nanning 530007, China;
2. Guangxi Crop Genetic Improvement and Biotechnology Laboratory, Nanning 530007, China
Solanum torvum is native to Latin America and the Caribbean, and is now widely distribted in Guangxi, Guangdong,Yunnan, Guizhou and Hainan Provinces of China. S. torvum is the closely wild relative of eggplant cultivar, which has good resistance to greensickness, bacterial wilt and rootknot nematode.Therefore,it is usually used as the stock of eggplant and tomato[1].During production, S.torvum shows the disadvantages of low germination rate, irregular germination,long seedling period, inconsistent growth stage of rootstock-scion and so on. Therefore, transgene should be used to indtroduce other good genes into S. torvum, to improve the botanical characteristics,and to enhance the productive value. Since an efficient and perfect plant regeneration system is the premise of crops improvement by genetic engineering, an efficient regeneration system should be established for S. torvum, which is of great importance for the studies on genetic transformation. Researches on S. torvum at home and abroad are mainly focused on the chemical components[2-4], seed germination[5-6]and grafting[7]. Few researches are found on the plant regeneration of S.torvum.Based on these, plant generataion of S. torvum was discussed in this research,with cotyledon of S.torvum as the explant; the optimal culture medium was screened,which layed a foundation for the genetic improvement.
Materials and Methods
Materials
S.torvum seeds were provided by Vegetable Research Institute,GuangxiAcademy of Agricutlural Sciences.
Methods
Seed sterilization Seeds were washed by tap water for 3-5 times,soaked in fresh water for 8-12 h,processed by 75% alcohol for 30 s, and sterilized by 0.1%mercuric chloride for 5-8 min.After washed by sterile water for 5-8 times, seeds were inoculated to MS culture medium for germination,which contained 30% sucrose and 0.7% agar. The cultivation condition was 25 ℃, 2 000 lx illumination intensity and 16 h/d illumination time.
Induction of callus and differentiation of adventitious buds Cotyledon was cut off from 10-12 d aseptic seedlings as explants. They were cut into 0.5 cm × 0.5 cm pieces, and inoculated to MS culture medium for induction containing different concentrations of hormones (Table 1). During inoculation, cotyledon should be rightside up with 5 pieces in each bottle.There were 12 bottles in each treatment. Induction status of adventitious buds was observed on 35 d. Callus rate=(Number of induced callus number/Number of inoculated explants) ×100%. Differentiation rate of adventitious bud = (Number of callus that differentiated dventitious buds / Number of total callus)×100%.
Induction of adventitious roots
When the induced adventitious buds grew to seedlings,the base was cut off and the seedlings were transferred into rooting medium. The rooting media included (1) MS + 0.1 mg/L IAA, (2)MS + 0.3 mg/L IAA and (3) MS + 0.5 mg/L IAA. Three plants were inoculated to one bottle, with 10 bottles for each treatment. After 15 d, rooting status was observed. Rooting rate =(Seedling number with roots/Inoculated seedling number)×100%.
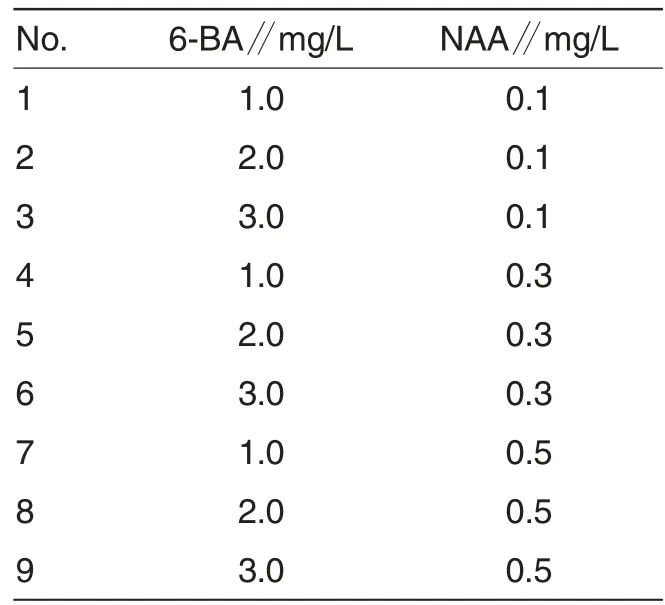
Table 1 Formula of differential medium
Transplanting and domestication of test-tube plantlets When regeneration seedlings became strong with developed root system, the bottle was opened for 3 d. Then, the seedlings were transferred to medium for moisturizing cultivation for 10-13 d, and then cultivated in the filed under normal management.
Results and Analyses
Effects of hormone combination on callus induction of S.torvum
On the 4thday after inoculation,leaves began to expand and became curled. On the 7thday, light green callus formed, whch was soft in texture.With the time prolonged, callus became large and compact; and the color turned to yellowish green. Table 2 reported that callus could be induced on all hormone combinations, but the inducing ability varied. Callus rates of treatments 4, 5 and 6 were the highest, reaching 100%. Callus in treatment 5 had the best growth status(1B); while treatment 2 had the lowest callus rate, which was only 85%. With the increase of NAA concentration, induction rate of S. torvum callus firstly enhanced and then reduced.Induction rate of S. torvum callus was the maximum in treatment 5 with the best growth status. Therefore, the optimal culture medimum for S. torvum callus was MS+0.3 mg/L NAA+2.0 mg/L 6-BA.
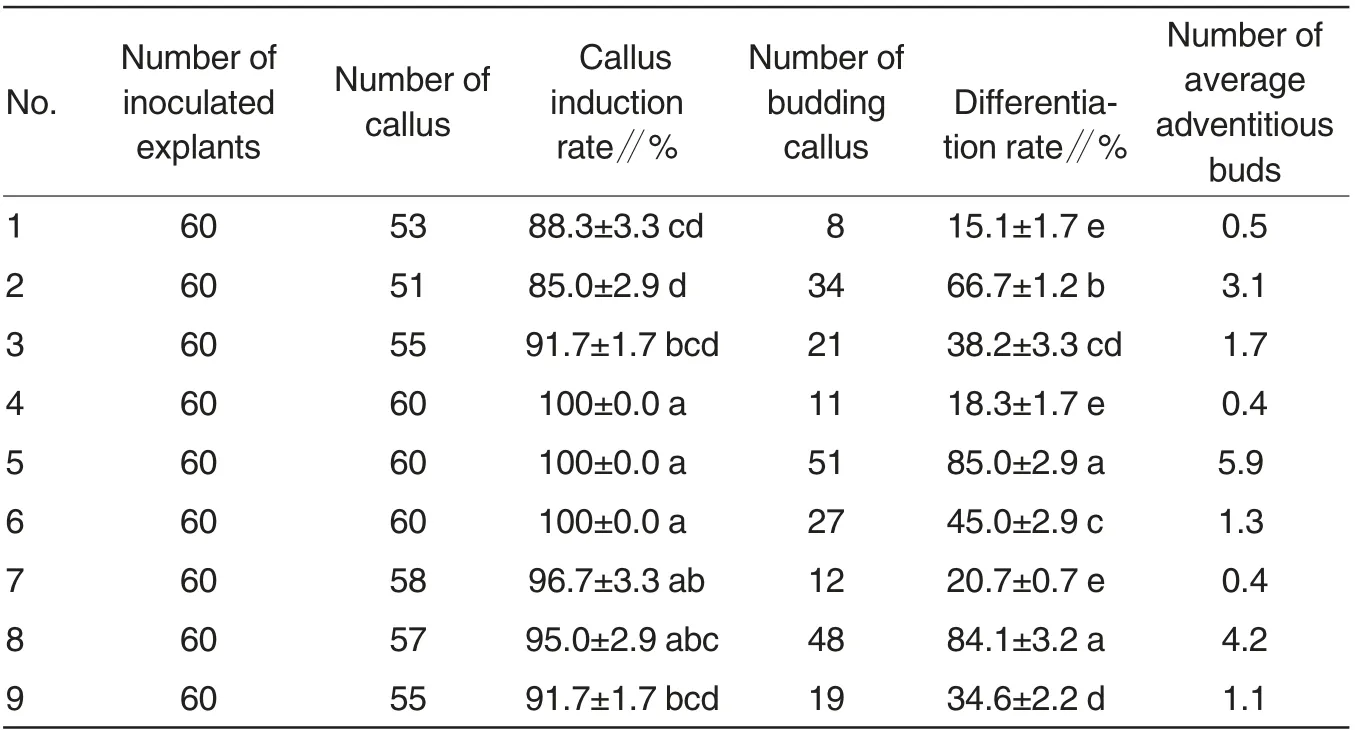
Table 2 Effects of hormone combination on callus induction and adventitious buds differentiation of S.torvum
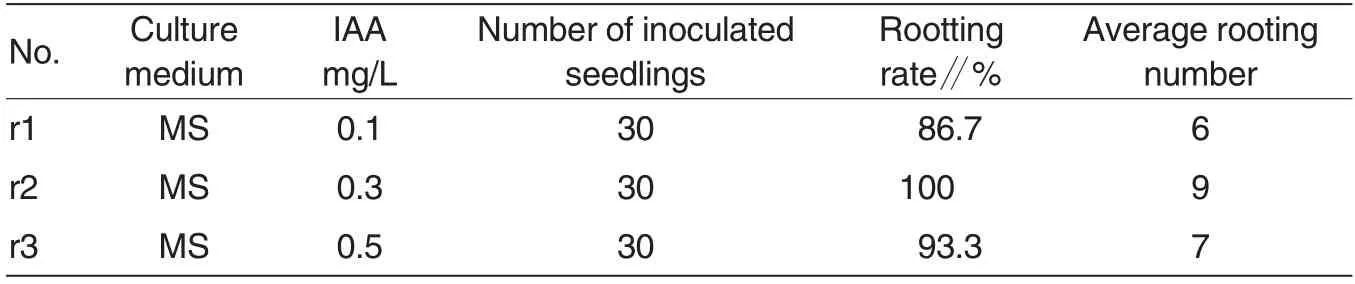
Table 3 Effects of hormone combination on roots induction of S.torvum
Effects of hormone combination on the adventitious bud induction of S.torvum
The time of adventitious bud induction varied under different hormone combinations. Buds appeared on 15 d after transplanting, which were green and compact. After the 18thday, buds gradually differentiated into seedlings.The laetest buds appeared on 18 d.Table 2-2 reported that as the 6-BA concentration increased,differentiation rate of explants adventitious buds firstly increased and then reduced. When 1.0 mg/L 6-BA and 0.1 mg/L NAA were added into MS culture medium,differentiation rate of explants adventitious buds was relatively low, which was only 15.1% . The adventitious buds had different growth trends and the growth speed was slow. When 2.0 mg/L 6-BA and 0.3 mg/L NAA were added into MS, the differentiation rate of adventitious buds of explants was 85.0% . There were 6 differentiated adventitious buds on average. The time for induction of adventitious buds was the shortest, and the adventitious buds were in the same size. The color was green (1C),and the buds gradually grew to seedlings (1D). Therefore,the optimal culture medium for adventitious buds induction of S.torvum was MS+2.0 mg/L 6-BA+0.3 mg/L NAA.
Effects of hormone combination on roots induction of S.torvum
The three culture mediums could all induce roots. Among them, earliest roots appeared on r2 culture medium.Seedlings were transplanted for rooting, and the roots appeared on the 4thday. Table 3 reported that when 0.3 mg/L IAA was added into culture medium,the rooting rate reached 100% .The average rooting number was 9,and there were many fibrous roots under this concentration. In other words,the total surface area of roots were relatively great and the absorption was relatively great(1E).r3 culture medium was adding 0.5 mg/L IAA into MS culture medium; the induction rate of adventitious roots reached 93.3%but the fibrous roots were relatively small. In the aspect of growth speed of roots,r2 also showed significant advantages,indicating that adding 0.3 mg/L IAA into IAA was helpful to the rooting and root system growth of S. torvum.Therefore it could be concluded that MS + 0.3 mg/L IAA was the optimal culture medium for adventitious roots S.torvum.
Transplanting
When the regenerated roots were strong,the bottle cap was opened for 2 d.After washing the culture mediun on roots, the seedlings were transferred into phytotrone and moisturized for about 4 d.Thus,strong seedlings were obtained (1F). The transplanting survival rate reached more than 90%.
Discussions and Conclusions
Proper high-frequency regeneration system should be established,which was an effective way for the reproduction of asexual tube seedlings and the storage of high-quality seeds,the basis to realize plant genetic transformation, and the key to the success of gene transformation. Reports had been found on the aspect of genus Solanum. With S. torvum segments as the explants, induction was carried out on MS+1.0 mg/L KT+0.01 mg/L IBA.The reproduction coefficient of adventitious buds was 2-3; induction was carried out on rooting medium 1/2MS+0.1 mg/L IBA.The rooting rate was 90% after 10 d; and the average rooting number per plant was 7-8[8].
Except S. torvum, one-step induction of callus and adventitious bud were also researched on Solanum mammosum and Rhizophora mucronata Poir[9-10]. All the reproduction coefficients of these testes were lower than those of this research, and the operation was more complex. At the same time, one-step induction of callus and adventitious bud of cotyledon were also researched for Rhizophora mucronata Poir; the experimental formula was MS + 2.0 mg/L 6-BA + 0.3 mg/L IAA[11]. Since NAA could be directly used for pot Sterilization while IAA was easily decomposed, 0.22 μm millipore filter should be used for filter sterilization. Therefore, this method was simple and had little pollution. In this research,a group of culture mediums with high induction rate of callus and adventitious bud were screened.The induction rates of callus and adventitious bud reached 100% and 85%, respectively. The number of average buds was 6; the induced callus and adventitious bud were compact and their color was yellowish green.These culture mediums enhanced the differentiation rate, reduced the contamination rate and research cost. In the later period of adventitious buds growth, some leaves showed albinismif they were not immediately transferred to rooting culture medium.These might be caused by insufficient illumination, nutritional deficiency and so on. Further research was still needed on the concrete reasons. In this research, an in vitro regeneration system was established with cotyledon of S. torvum aseptic seedling as the explants, which provided an effective way for the rapid reproduction of S. torvum, and laid a foundation for genetic transformation.With cotyledon of S.torvum as the explants, 2.0 mg/L 6-BA and 0.3 mg/L NAA were added into MS culture medium, which could induce the callus and adventitious bud.The induction rates of callus and adventitious bud reached 100% and 85% , respectively. Therefore, this culture medium could induce the formation of callus formation and the differentiation of adventitious bud, which reduced both contamination rate and research cost.
[1]HAO J(郝晶),ZHOU BL(周寶利),LIU N(劉娜), et al. Relationship between the resistance to verticillium wilt and the microbial biomass and soil enzyme in rhizosphere soil of grafted eggplant (嫁接茄子抗黃萎病特性與根際土壤微生物生物量和土壤酶的關系). Journal of Shenyang Agricultural University (沈陽農(nóng)業(yè)大學學報),2009,40(2):148-151.
[2]SHU WH(舒?zhèn)セ?, ZHOU GX(周光雄),YE WC(葉文才).Chemical components of Solanum torvum (水茄的化學成分研究).Chinese herbal medicine (中草藥),2011,42(3):424-427.
[3]Alida P C, Luis B C, Anne C M, et al.Steroidal saponins from the fruits of Solanum torvum Phytochemistry, 2013,86,137-143
[4]Barbosa P S,Camara A G,Silva T M,et al. Chemical constituents of essential oils from Solanum torvum leaves,stems, fruits, and roots. Chemistry of Natural Compounds, 2012, 48(4): 698-699
[5]QIAN ZW(潛宗偉),WU Z(吳震), CHEN HL (陳海麗), et al. Effects of induction treatment on the germination of wild Solamum torvum (不同引發(fā)處理對野生茄子砧木托魯巴姆萌發(fā)的影響).Seeds(種子),2009,28(6):12-16.
[6]LIU FH(劉鳳華),LI L(李蕾),YUN XF(云興福). Effects of chemical compounds on the germination of wild Solamum torvum (化學物質對野生茄子托魯巴姆種子萌發(fā)的影響). Inner Mongolia Agricultural Science and Technology (內(nèi)蒙古農(nóng)業(yè)科技),2011(2):43-47.
[7]HU YJ(胡永軍),YANG MK(楊茂坤),XIA WY(夏文英).Technology of grafting the resistant Solamum torvum to control tomato root-knot eelworm (抗性砧木托魯巴姆嫁接控制番茄根結線蟲技術).Greenhouse horticulture (溫室園藝),2007(1):40-41.
[8]ZHANG H (張紅). Tissue culture and rapid propagation techniques of Solanum torvum (水茄的組織培養(yǎng)和快速繁殖). Plant Physiology Communications(植物生理學通訊),2010,46(11):1175-1176.
[9]HAO AP (郝愛平), WEI JC (魏繼承).Rapid propagation technology of onestep-seedlings formation of Solanum mammosum leaves(乳茄葉片一步成苗組培快繁技術研究)[J]. Northern Horticulture(北方園藝),2010,07:122-123.
[10]HAO AP (郝愛平), WEI JC (魏繼承),GUO HY(國會艷).Technique of tissue culture and rapid propagation of Solana integrilolium Poir (非洲紅茄組培快繁技術研究)[J].Northern Horticulture(北方園藝),2009,12:112-113.
[11]FANG YY (方巖巖),WANG YK (王益奎),WANG H(王紅),et al.Adventitious bud induction and plant regeneration of cotyledon of Rhizophora mucronata Poir(紅茄子葉的不定芽誘導及植株再生)[J]. Journal of Southgern Agriculture(南方農(nóng)業(yè)學報),2013,05:751-754.
 Agricultural Science & Technology2015年10期
Agricultural Science & Technology2015年10期
- Agricultural Science & Technology的其它文章
- Research Advances in Gene Regulation and Genetic Improvement of Fish Feeding
- Instrucions for Authors
- Cambridge Scientific Abstracts (CSA)
- Overview of Pharmaceutical Research on the Poria with Hostwood of Traditional Chinese Medicine
- Molecular Marker Assisted Selection for Fusarium Wilt Resistance Breeding in Watermelon(Citrullus lanatus)
- Study on Relative Soil and Water Conservation Benefits of Ridge Tillage in Different Terrain Conditions in the Black Soil Area of Northeast China
