Effects of frugivorous birds on seed retention time and germination in Xishuangbanna, southwest China
Ting-Ting SHI, Bo WANG, Rui-Chang QUAN
?
Effects of frugivorous birds on seed retention time and germination in Xishuangbanna, southwest China
Ting-Ting SHI, Bo WANG, Rui-Chang QUAN*
Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Xishuangbanna Yunnan 666303, China
The dispersal of many plants depends on transportation by birds as seed dispersers. The birds play an important role in long distance seed dispersal and may also affect seed germination. However, for plants who have many bird dispersers, the influence of dominant and non-dominant dispersers on retention time (dispersal distance) and germination remains poorly understood. In this study, we performed experiments with captive frugivorous birds and fruiting plant species to study the effects of dominant and non-dominant dispersers on seed retention time (SRT) and germination (seed germination percentage and germination speed). Our study showed a great interspecific variation in the effects of frugivorous birds on both SRT and germination. Some birds enhance the germination of a given plant species, but others do not. Generally, the dominant visitors improved the seed germination and performed longer seed retention time.
Dominant visitors; Frugivores; Interaction; Seed germination; Xishuangbanna
INTRODUTION
Seed dispersal by frugivores is a critical ecosystem process found throughout the world (Jordano, 2000; Schleuning et al, 2011). Benefits of seed dispersal include avoiding inbreeding depression as well as avoiding a high density of predators and pathogens near their parent trees (Janzen-Connell hypothesis) (Bell et al, 2006; Connell, 1971; Janzen, 1970; Mangan et al, 2010; Swamy et al, 2011). Seed retention time (SRT), the time elapsed from fruit ingestion to seed elimination/defecation (Fukui, 2003), dictates the effective seed dispersal distance (Wotton et al, 2008). Some researchers have shown that SRT usually varies among different plant species due to differences in seed size (Chang et al, 2012; Figuerola et al, 2010; Fukui, 2003). There is also a positive correlation between SRT and the body size of the frugivores (Murphy et al, 1993; Spiegel & Nathan, 2007). However, comparing SRT between different bird species has been rarely studied, and existing studies have produced inconsistent results. For instance, Spiegel & Nathan (2007) reported that the SRT ofconsumed by Tristram’s grackle () was much longer than those seeds consumed by Yellow-vented bulbul (). On the other hand, Figuerola et al (2010) showed no differences between the SRT for the plantsandingested by four waterfowl species.
Apart from avoiding potential Janzen-Connell effects, frugivores can also change seed germination success through the digestive processing of the seed. Many studies have shown that the digestive systems of frugivores significantly influence seed germination (Robertson et al, 2006; Samuels & Levey, 2005; Traveset & Verdú, 2002). For example, digestion of frugivores can either increase (Reid & Armesto, 2011) or decrease (Lieberman & Lieberman, 1986) the percent of seed germinated or the germination speed (and can reduce predator detection and consumption of seeds as well as attacks by pathogens) (Fricke et al, 2013). The effects of gut passage on seed germination usually differs between pairs of bird and plant species (Barnea et al, 1991; Traveset et al, 2001b).
Frugivorous birds are considered to be important seed dispersers in ecosystem processes (Shanahan et al, 2001). The interaction between frugivorous birds and fruiting plants has been examined in a range of studies (Jordano, 1995). But the relationship between frugivorous birds and fruiting plants has often been asymmetrical (Mccann et al, 1998; Paine, 1992) and not all frugivores are effective dispersers to the plant species consumed (Bradford & Westcott, 2011). For example, for a given species of plant, not all the visiting birds but only one or two bird species had a mutualistic and compact relationship with it (Silveira et al, 2012). As such, in our study, we classified birds into “dominant” and “non-dominant” visitors according to their observed visiting frequency to seven plant species. Then we compared the different effects between the two groups of birds on SRT and seed germination for these plant species.
MATERIALS AND METHODS
Study areas and species
This study was conducted in Xishuangbanna Tropical Botanical Garden (570 m a.s.l., centered at N21°55′, E101°16′), in Yunnan Province, southwest China. Our study area lies within the Indo-Burma biodiversity hotspot and contains a high diversity of fruiting plant species (Myers et al, 2000). Four-hundred-thirty-four bird species have been recorded in this region, constituting 36.3% of the avian richness in China (Jiang et al, 1998).
To better understand the effect of dominant birds and non-dominant birds on fruiting plants, we selected seven common fleshy fruited species that are visited by birds and non-avian frugivores (Table 1). The seeds of one of the species,, were divided into two types:(big) and(small), as their fruits were significantly different in size (mean±, 9.19±0.55 mm8.19±0.47 mm,=7.608,<0.001). To minimize the intraspecific variation in fruit features, fruits were collected from either a single mother tree for woody plants or a cluster of individuals for herbaceous plants within the same day (Fukui, 2003).
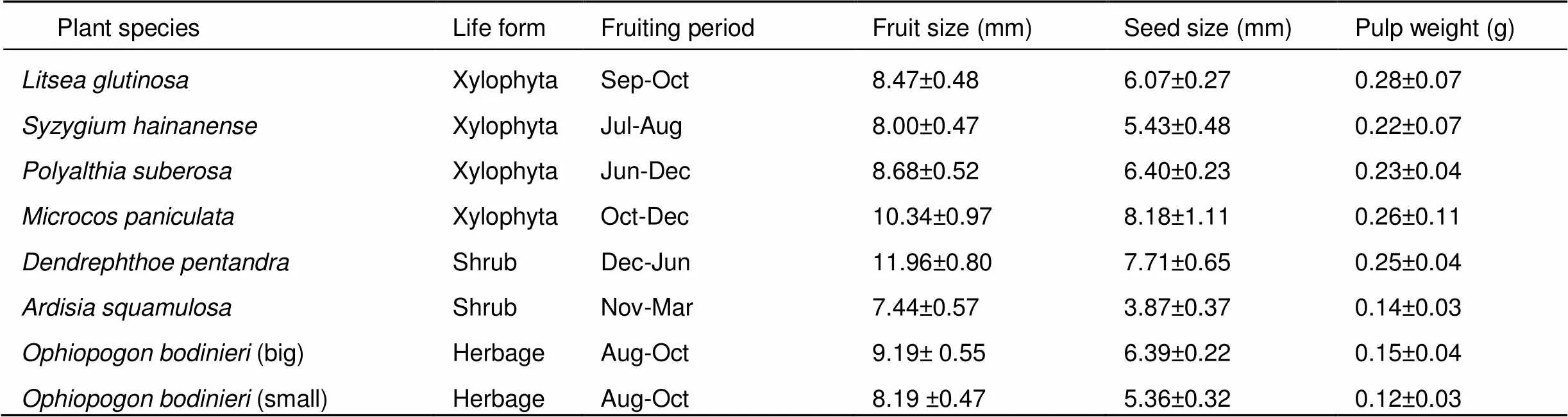
Table 1 Fruiting period and fruit traits of our study species
Six common frugivorous bird species were selected for the experiment: Red-whiskered Bulbul (), Sooty-headed Bulbul (), Black-crested Bulbul (), Blue-throated Barbet (), Plain Flowerpecker (), and Crested Myna (). These species were selected because they are the most common and abundant species in our study site, and consume large amounts of fruits from a wide variety of plant species (Yang, 1994, 2004). All individuals were kept in separate cages (30 cm×30 cm×40 cm, length×width×height) and fed daily diets consisting of apple, pear, bananas, and mealworms. Water was suppliedthroughout the experiment. All Plain Flowerpecker individuals were released at the beginning of the experiment because they did not consume any fruits in captivity.
Field observation
Field observations were conducted from July 2012 to July 2013, during the fruiting period of the seven target species. Three trees or clusters for each plant species were selected to record the number of individuals of each bird species that visiting the plant species; field observation was carried out at peak times of avian activity(0700-0830h, 1600-1730h). During the observation, the entire tree or cluster of plants was scanned once every 10 minutes with a 10×42 binocular (Olympus). Each tree or cluster was observed for 3 days in total with 3 hours in each day. We defined the dominant visitors as the species with the largest number of individuals during the observation period.
Seed retention time
Thirty-three individuals of birds were used in the SRT experiment, including 8 Red-whiskered Bulbuls, 6 Sooty-headed Bulbuls, 8 Black-crested Bulbuls, 5 Blue-throated Barbets and6Crested Mynas, respectively. To ensure that birds would consume fruit from the start of the trial onwards, and thereby standardize the SRT, the maintenance diet was removed the night before trials (Tewksbury et al, 2008). The experiments began in the morning (0800h) and the birds were given 30 fruits of a single plant species. The fruits were subsequently removed 10 minutes after the first fruit was eaten and a moveable plastic tray was placed at the bottom of the cage to collect the seeds defecated every 5 minutes (Spiegel & Nathan, 2007). The experiment ended when no seed was observed in the faeces for 60 minutes. We used the midpoint of the first 10 free feeding minutes (5 minutes after the first fruit was eaten) as the beginning of the test. The mean SRT for each fruit species was defined as the mean retention time for all defecated seeds from a single bird.
Seed germination
Twenty fruits of each plant species were given to each bird. In total, 7 plant species, 4-5 bird species, and 5 individuals for each bird species were used for this experiment. After all of the seeds of the 20 fruits were excreted, they were extracted from the faeces and dried at room temperature. Some individuals of Crested Mynaappeared ill during the experiment, and they were released before the experiment ended. Therefore, for the myna we have consumption and germination trials for 3 plant species only (,and). Furthermore, the seeds ofwere collected from defecations of Plain Flowerpeckerin the field. Collecting these seeds/defecations in the field was possible because our field observation showed that the flowerpecker faeces (includingseeds) is easily identified and found. We also conducted a control treatment for each plant species by removing the fruit pulp manually. To standardize the manual removal with the bird digestion treatments, there were 5 repetitions in the control treatments and 20 seeds in each repetition.
Seeds were placed in Petri dishes with agar medium in a constant temperature incubator (27oC, 14 h light, 10 h darkness) (Reid & Armesto, 2011). Seeds that germinated were counted and removed daily to reduce their possible effects on non-germinated seeds (Mandon-Dalger et al, 2004). Seeds were considered geminated when the radicle emerged (Traveset et al, 2001a). Seed germination checks would stop when no seeds germinated for 2 consecutive months. These non-germinated seeds were all unviable due to fungus infection or rot. Two indices were introduced to estimate the germination differences between the different treatments (digested by various bird species and control seeds): final germination percentage (GP) and germination speed (GS). The indices were computed are as follows:
GP=(N1+N2+˙˙˙+Nn)/20×100% (1)
GS=N1/1+N2/2+˙˙˙+Nn/n (2)
Where, Nnexpresses the number of germinated seeds in day.
Data analysis
A one-way ANOVA with Tukey’s honest significant difference (HSD) multiple comparsions was used to analyze the differences of GP, GS and mean SRT among various bird species for each plant species. A square root transformation was applied to make the mean SRT values exhibit homoscedasticity and normality. All analyses were performed in R 3.1.1 (The R Core Team, 2014).
RESULTS
Visiting frequency of birds to each plant species
There were 27 hours of observation for each plant species and 216 (becausewere divided into two types) hours in all. Eleven species and 1 021 individuals of bird were observed in total. No birds visited(small and big) andTheRed-whiskered Bulbul was the dominant visitor of,,andwith visiting frequencies of 80.34%, 43.33%, 45.61% and 79.17%, respectively (Figure 1)The Plain Flowerpeckerwas the dominant visiting species for(75.25%) (Figure 1).
Figure 1 Frequency of different visitors to each plant species
Seed retention time
Overall, 181 experiments of 7 plant species were conducted; this was less than anticipated because not all the plant seeds were eaten within the regulation time (10 minutes) by some ofthe bird species (Appendix 1). The mean SRTs varied significantly between bird species for the plants(4, 21=6.64,=0.001),(4, 28=3.37,=0.023),(big) (3, 18=4.08,=0.023) and(small)(3, 18=4.25,=0.020), but not for(3, 21=1.42,=0.266)(3, 20=2.19,=0.121) and(4, 24=1.37,=0.274) (Figure 2). Seeds ofanddigested by their dominant visitors showed a longer mean SRT (Figure 2C, D).
Seed germination
No seeds ofandgeminated from any treatment. The GP significantly differed among treatments for both(5, 24=4.90,=0.003) and(5, 24=64.8,<0.001) (Figure 3B, C). No differences in GP were found between treatments for(4, 20=0.80,=0.539)(big) (4, 20=0.37,=0.825)(small) (4, 20=2.16,=0.111) and(5, 24=1.24,=0.323) (Figure 3A, D, E, F). For, the dominant visitors (i.e. Red-whiskered Bulbul) significantly increased GP, and some non-dominant species (Crested Myna and Black-crested Bulbul)also improved GP(Figure 3B). For, the GPof seeds digested by the dominant visitors was significantly larger than that of any other treatments (Figure 3C).
The GS differed significantly between treatments for both(5, 24=3.94,=0.010)and(5, 24=106.20,<0.001) (Figure 4B, C). No differences of GS were found among treatments for(4, 20=1.85,=0.159)(big) (4, 20=0.35,=0.842)(small) (4, 20=2.64,=0.064)and(5, 24=1.10,=0.386) (Figure 4). The GS ofwas enhanced by both thedominant visitors (Red-whiskered Bulbul) and some other non-dominant species (Crested Myna and Black-crested Bulbul) (Figure 4B). For, the GS of seeds digested by the dominant visitors was significantly larger than any other treatments (Figure 4C).
Figure 2 Seed retention time of the six plant species
A:; B:; C:; D:; E:(big);F:(small);G:; Different lowercase letters indicate a significant difference (≤0.05); Black bars represent the dominant visitor for the given plant species.
Figure 3 Germination percentage of digested and control seeds of the five plant species
A:; B:; C:; D:(big);E:(small);F:; Different lowercase letters indicate a significant difference (≤0.05); Black bars represent the dominant visitor for the given plant species.
Figure 4 Germination speed of digested and control seeds of the five plant species
A:; B:; C:; D:(big);E:(small);F:; Different lowercase letters indicate a significant difference (≤0.05); Black bars represent the dominant visitor for the given plant species.
DISCUSSION
Previous studies have usually chosen one or a few species of birds that consume a particular plant species and have then compared differences between these bird species on SRT and germination (Barnea et al, 1991; Charalambidou et al, 2003; Figuerola et al, 2010; Fukui, 2003; Jordaan et al, 2011). However, the effects of birds on plants varies between both bird and plant species (Traveset & Willson, 1997; Yagihashi et al, 1999). As such, our study considered a range of avian frugivore and plant species pairs.
In general, the process of seed passage through the avian digestive tract is a key aspect of endozoochory, and the SRT is a significant factor for both plants and birds (Fukui, 2003). Our results showed that SRT exhibited significant variation between avian frugivores visiting four plant species (,,(big)(small)), similar to previous studies of Jordaan et al (2011), but inconsistent with the result of Figuerola et al (2010). The variation of SRT may be due to differences in disperser body size (Spiegel & Nathan, 2007), seed size consumed (Figuerola et al, 2010; Fukui, 2003), or some combination thereof. Some studies have suggested that seed dispersal distance is a function of SRT (Fukui, 2003). Thus, the variation of SRT among bird species may lead to wide variation in seed dispersal distance.
Once ingested by birds, seeds may experience both chemical and mechanical processing in the digestive system. As a result, the seed coats or endocarp may be altered, thereby affecting germination (Barnea et al, 1990). However, whether digestion increases seed germination is unclear (Traveset, 1998). In our study, digestion significantly affected both GP and GS for some species but not for others. For plants which had bird visitors in the field (,), both GP and GS were improved through bird digestion. For the three species which had no bird visitors in the field ((big)(small)), the GP and GS were not influenced by birds ingesting. For the plant species that have bird consumers, it looks that birds are not only seed dispersers but also germination enhancers, suggesting that their relationship is a mutualism.
By dividing the visitors into dominant and non-dominant ones, we found that the SRT of a given plant species digested by dominant visitors was either the same as or longer than that comsumed by non-dominant visitors. The GP and GR forandwere much higher after consumption by the dominant visitors than by some non-dominat visitors as well as control ones. Therefore, compared to non-dominant visitors, the action on seed retention time and germination of dominant visitors is more prominent. Perhaps the different effects on seed germination and retention time by dominant and non-dominant visitors can also help to explain why some previously studies reported inconsistent results.
In conclusion, this study supports findings in the literature that frugivorous birds have positive effects on seed retention time and ultimately seed germination. Moreover, dominant visitors seem to confer more benefits than non-dominant visitors to a plant species, suggesting that future studies should consider the visiting frequency of frugivorous birds as an important ecological factor when studying gut passage time of seeds and how birds affect seed germination.
ACKNOWLEDGEMENTS
We thank Shan-Wen SUN from University of Bayreuthfor statistical suggestion and helping with the experiment. The manuscript was improved bycomments from Ming-Xia ZHANG (Xishuangbanna Tropical Botanical Garden), Charlotte CHANG (Princeton University) and Eben GOODALE (Guangxi University).
REFERENCES
Barnea A, Yom-Tov Y, Friedman J. 1990. Differential germination of two closely related species of solanum in response to bird ingestion.57(2): 222-228.
Barnea A, Yom-Tov Y, Friedman J. 1991. Does ingestion by birds affect seed germination?.5(3): 394-402.
Bell T, Freckleton RP, Lewis OT. 2006. Plant pathogens drive density-dependent seedling mortality in a tropical tree.9(5): 569-574.
Bradford MG, Westcott DA. 2011. Predation of cassowary dispersed seeds: is the cassowary an effective disperser?.6(3): 168-177.
Chang SY, Lee YF, Kuo YM, Chen JH. 2012. Frugivory by Taiwan Barbets () and the effects of deinhibition and scarification on seed germination.90(5): 640-650.
Charalambidou I, Santamaría L, Langevoord O. 2003. Effect of ingestion by five avian dispersers on the retention time, retrieval and germination ofseeds.17(6): 747-753.
Connell JH. 1971. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees.: den Boer PJ, Gradwell G. Dynamics of Populations. Wageningen: PUDOC, 298-312.
Figuerola J, Charalambidou I, Santamaria L, Green AJ. 2010. Internal dispersal of seeds by waterfowl: effect of seed size on gut passage time and germination patterns.97(6): 555-565.
Fricke EC, Simon MJ, Reagan KM, Levey DJ, Riffell JA, Carlo TA, Tewksbury JJ. 2013. When condition trumps location: seed consumption by fruit-eating birds removes pathogens and predator attractants.16(8): 1031-1036.
Fukui A. 2003. Relationship between seed retention time in bird's gut and fruit characteristics.2(1): 41-48.
Janzen DH. 1970. Herbivores and the number of tree species in tropical forests.104(940): 501-528.
Jiang WG, Wen XJ, Yang XJ, Yang L. 1998. The first report on bird diversity in Xishuangbanna.19(4): 282-288. (in Chinese)
Jordaan LA, Johnson SD, Downs CT. 2011. The role of avian frugivores in germination of seeds of fleshy-fruited invasive alien plants.13(8): 1917-1930.
Jordano P. 1995. Angiosperm fleshy fruits and seed dispersers: a comparative analysis of adaptation and constraints in plant-animal interactions.145(2): 163-191.
Jordano P. 2000. Fruits and frugivory.: Fenner M. Seeds: the Ecology of Regeneration in Plant Communities. Wallingford: CABI, 125-166.
Lieberman M, Lieberman D. 1986. An experimental study of seed ingestion and germination in a plant-animal assemblage in Ghana.2(2): 113-126.
Mandon-Dalger I, Clergeau P, Tassin J, Rivière JN, Gatti S. 2004. Relationships between alien plants and an alien bird species on Reunion Island.20(6): 635-642.
Mangan SA, Schnitzer SA, Herre EA, Mack KM, Valencia MC, Sanchez EI, Bever JD. 2010. Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest.466(7307): 752-755.
Mccann K, Hastings A, Huxel GR. 1998. Weak trophic interactions and the balance of nature.395(6704): 794-798.
Murphy SR, Reid N, Yan ZG, Venables WN. 1993. Differential passage time of mistletoe fruits through the gut of honeyeaters and flowerpeckers: effects on seedling establishment.93(2): 171-176.
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities.403(6772): 853-858.
Paine RT. 1992. Food-web analysis through field measurement of per capita interaction strength.355(6355): 73-75.
Reid S, Armesto JJ. 2011. Avian gut-passage effects on seed germination of shrubland species in Mediterranean central Chile.212(1): 1-10.
Robertson AW, Trass A, Ladley JJ, Kelly D. 2006. Assessing the benefits of frugivory for seed germination: the importance of the deinhibition effect.20(1): 58-66.
Samuels IA, Levey DJ. 2005. Effects of gut passage on seed germination: do experiments answer the questions they ask?.19(2): 365-368.
Schleuning M, Blüthgen N, Fl?rchinger M, Braun J, Schaefer HM, B?hning-Gaese K. 2011. Specialization and interaction strength in a tropical plant-frugivore network differ among forest strata.92(1): 26-36.
Shanahan M, So S, Compton SG, Corlett R. 2001. Fig-eating by vertebrate frugivores: a global review.76(4): 529-572.
Silveira FAO, Mafia PO, Lemos-Filho JP, Fernandes GW. 2012. Species-specific outcomes of avian gut passage on germination of Melastomataceae seeds.145(3): 350-355.
Spiegel O, Nathan R. 2007. Incorporating dispersal distance into the disperser effectiveness framework: frugivorous birds provide complementary dispersal to plants in a patchy environment.10(8): 718-728.
Swamy V, Terborgh J, Dexter KG, Best BD, Alvarez P, Cornejo F. 2011. Are all seeds equal? Spatially explicit comparisons of seed fall and sapling recruitment in a tropical forest.14(2): 195-201.
Tewksbury JJ, Levey DJ, Huizinga M, Haak DC, Traveset A. 2008. Costs and benefits of capsaicin-mediated control of gut retention in dispersers of wild chilies.89(1): 107-117.
The R Core Team. 2014. R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing.
Traveset A. 1998. Effect of seed passage through vertebrate frugivores' guts on germination: a review.1(2): 151-190.
Traveset A, Riera N, Mas R. 2001a. Ecology of fruit-colour polymorphism in Myrtus communis and differential effects of birds and mammals on seed germination and seedling growth.89(5): 749-760.
Traveset A, Riera N, Mas RE. 2001b. Passage through bird guts causes interspecific differences in seed germination characteristics.15(5): 669-675.
Traveset A, Verdú M. 2002. A meta-analysis of the effect of gut treatment on seed germination.: Levey D, Silva W, Galetti M. Seed Dispersal and Frugivory: Ecology, Evolution and Conservation. Wallingford: CABI, 339-350.
Traveset A, Willson MF. 1997. Effect of birds and bears on seed germination of fleshy-fruited plants in temperate rainforests of southeast Alaska.80(1): 89-95.
Wotton DM, Clout MN, Kelly D. 2008. Seed retention times in the New Zealand pigeon ().32(1): 1-6.
Yagihashi T, Hayashida M, Miyamoto T. 1999. Effects of bird ingestion on seed germination of twospecies with different fruit-ripening seasons.14(1): 71-76.
Yang L. 1995. The Avifauna of Yunnan, China (I). Kunming: Yunnan Science and Technology Press. (in Chinese)
Yang L. 2004. The Avifauna of Yunnan, China (II). Kunming: Yunnan Science and Technology Press. (in Chinese)
Appendix 1 The effective sample size of SRT experiment
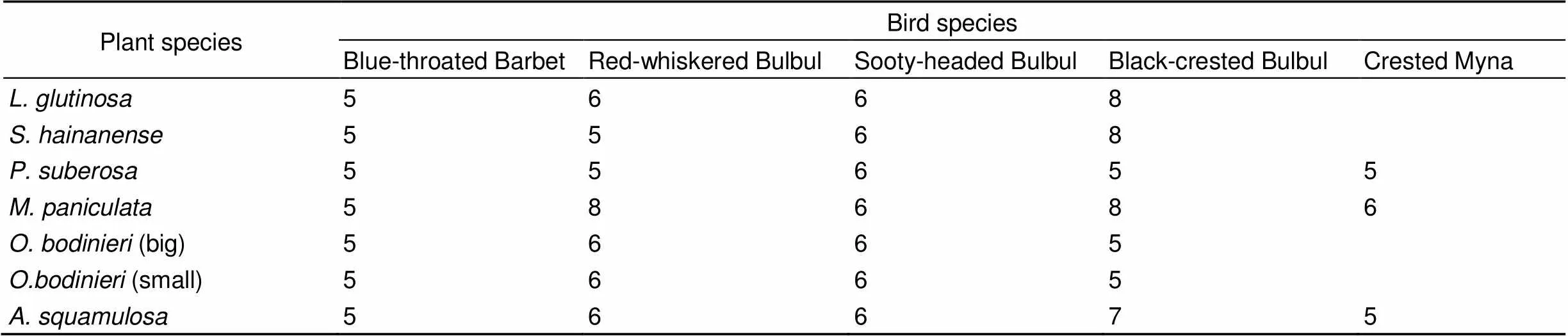
Plant speciesBird species Blue-throated BarbetRed-whiskered BulbulSooty-headed BulbulBlack-crested BulbulCrested Myna L. glutinosa5668 S. hainanense5568 P. suberosa55655 M. paniculata58686 O. bodinieri (big)5665 O.bodinieri (small)5665 A. squamulosa56675
Received: 09 March 2015; Accepted: 20 May 2015
This study was supported by funding from the National Nature Science Foundation of China (31370452) and the Chinese Academy of Science (KSCX2-EW-Q-17).
,E-mail: quanrc@xtbg.ac.cn
10.13918/j.issn.2095-8137.2015.4.241
- Zoological Research的其它文章
- Extraction and identification of membrane proteins from black widow spider eggs
- The breeding biology of Red-Whiskered Bulbul (Pycnonotus jocosus) in Xishuangbanna, southwest China
- Population genetic studies in the genomic sequencing era
- Why do we study animal toxins?
- Zoological Research is recognized as a core journal by the Research Center of Chinese Science Evaluation (RCCSE)
- The internationalization voyage of Zoological Research

