The neuroprotective and regenerative potential of parkin and GDNF/ Ret signaling in the midbrain dopaminergic system
The neuroprotective and regenerative potential of parkin and GDNF/ Ret signaling in the midbrain dopaminergic system
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease. The etiology of PD is still not completely understood, but the degeneration of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc), loss of DA innervation of the striatum, and protein aggregates in the form of Lewy bodies and neurites are its established hallmarks. In addition to α-synuclein accumulation in Lewy bodies and neurites, genetic mutations in the genes encoding parkin, PINK, DJ-1, LRRK2 and other proteins are associated with the inherited form of PD. An association study linked also the receptor tyrosine kinase Ret to PD (Meka et al., 2015). Currently there are only symptomatic treatments available for PD but no cure. Consequently much eff ort is being made to fi nd neurotrophic and other factors able to stimulate SNpc DA neuron protection and regeneration.
GDNF/Ret signaling promotes neuroprotection and regeneration: Glial cell line-derived neurotrophic factor (GDNF) is the founding member of the GDNF family of ligands (GFLs), which include neurturin, artemin and persephin belonging to the transforming growth factor-β (TGF-β) superfamily (Airaksinen and Saarma, 2002). GDNF binds with high affinity to the GDNF family receptor α1 (GFRα1) activating the receptor tyrosine kinase Ret and other GDNF receptors such as the neuronal cell adhesion molecule NCAM, integrins αV and βI, syndecan-3 or N-cadherin (Airaksinen and Saarma, 2002; Paratcha and Ledda, 2008). The canonical GDNF receptor Ret signals through many pathways such as PI3K/Akt, Ras/MAPK, NF-κB, JNK, and PLCγ/PKC, while NCAM can activate Fyn and FAK (Airaksinen and Saarma, 2002; Aron and Klein, 2011). GDNF is thought to be the most potent neurotrophic factor for the two midbrain dopaminergic (DA) neuron populations, the SNpc DA neurons that die in PD patients and the ventral tegmental area (VTA) DA neurons altered during drug addiction (Aron and Klein, 2011). Surprisingly, although GDNF stimulates neuronal diff erentiation, neurite outgrowth, synapse formation, and dopamine release in cell culture (Airaksinen and Saarma, 2002; Paratcha and Ledda, 2008), it was shown to be dispensable for DA neuron development and maintenance (Kopra et al., 2015). In contrast, Ret is dispensable during development but important for maintaining SNpc DA neurons in aging mice as we showed in two diff erent DA system specifi c Ret knockout mouse line (Kramer et al., 2007; Meka et al., 2015).
In toxin induced PD animal models such as 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treated mice, rats and monkeys, GDNF can provide very effi cient neuroprotection of the midbrain DA cell bodies and axons and even stimulates regeneration of the remaining axons over time (Airavaara et al., 2012). Unfortunately, clinical trials using GDNF or neurturin on PD patients have so far been inconclusive (Airavaara et al., 2012). We showed that while Ret-defi cient mice are not more susceptible to MPTP-induced toxicity, the DA neuron axons do not resprout in the striatum of these mice after MPTP-induced DA system degeneration (Kowsky et al., 2007). Thus, the Ret receptor seems to be essential for mediating axonal regeneration function in DA neurons of endogenous GDNF. Since besides Ret, DA neurons express all four alternative GDNF receptors, and GDNF protein levels are rather low in adult mice, further experiments are needed to address the question of which GDNF receptor mediates neuroprotective and regenerative eff ects of GDNF on midbrain DA neurons.
The efficacy of GDNF in protecting DA neurons from various kinds of stress - such as protein aggregation, ubiquitin proteasome system dysfunction and endoplasmatic reticulum (ER) stress - known to play a critical role in PD pathogenesis is poorly studied (Ulusoy and Kirik, 2008). In a rat model of PD with viral overexpression of α-synuclein GDNF was shown not to play a protective role in DA neuron degeneration (for references see (Decressac et al., 2012)). The authors propose that α-synuclein accumulation down-regulates the transcription factor Nurr1 which leads to a reduced expression level of the Nurr1-regulated GDNF receptor Ret (Decressac et al., 2012). It is still under debate to what degree Ret might be down-regulated in PD patients with Lewy bodies and neurites due to accumulation of α-synuclein. However, since Ret is still detectable in these PD patients, its down-regulation is most likely not the main reason why Ret ligands so far have not shown benefi cial eff ect in clinical PD trials.
Parkin overexpression promotes neuroprotection and perhaps regeneration: The E3 ubiquitin protein ligase parkin has been found to be mutated in some familiar forms of PD. Parkin has a wide neuroprotective function that can be mediated by promoting mitochondrial integrity, increasing proteasomal degradation of toxic substances, and/or modulating non-degradative ubiquitin signaling within cell death or viability pathways (Winklhofer, 2014). Parkin, together with the PD associated protein PINK1, was shown to initiate the removal of damaged mitochondria by mitophagy. Parkin also acts upon its substrate aminoacyl-tRNA synthetase complex interacting multifunctional protein-2 (AIMP2) by ubiquitination for proteasomal degradation to prevent cell death induction. Another parkin substrate termed parkin-interacting substrate (PARIS) acts as a transcriptional repressor of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), which stimulates mitochondrial biogenesis as a coactivator of various transcription factors such as nuclear respiratory factor (NRF)-1 and -2. Furthermore, parkin activates NF-κB signaling by binding to the linear ubiquitin assembly complex (LUBAC), which contains two E3 ligases, HOIP and HOIL-1L, that mediate linear ubiquitination of NEMO (Winklhofer, 2014).
Parkin overexpression showed a mild neuroprotective eff ect in MPTP and 6-OHDA animal models of PD, but it was much less efficient compared to GDNF (Ulusoy and Kirik, 2008). Parkin has been shown to be upregulated by various stress conditions and seems to be protective against mitochondrial and ER stress, proteotoxic stress, excitotoxicity, proapoptotic stimulation, and overexpression of toxic, misfolded protein species such as α-synuclein (Winklhofer, 2014). Under mitochondrial stress conditions parkin may stabilize mTOR by ubiquitination, thereby allowing mTOR complex 1 (mTORC1) activity stimulating mitochondrial biogenesis and cell survival. However, in a negative feedback loop mTORC1 activity might down-regulate parkin expression. Like Ret knockout mice, parkin-deficient mice do not seem to be not more sensitive to MPTP toxicity compared to wildtype mice (Ulusoy and Kirik, 2008).
So far, there are no reports addressing the question if parkin might also be able to promote neuroregeneration. Parkin is responsible for proper mitochondrial function and transport required for axon growth. In order to stimulate axon outgrowth and attraction, intracellular calcium levels need to rise. Parkin stimulates calcium release from the endoplasmatic reticulum bystimulating phospholipase Cγ1 (PLCγ1). Akt and MAPK signaling have been shown to be involved in axon outgrowth promotion, which is not only activated by Ret but also by parkin. Clearly more experiments are needed to defi ne the full potential of parkin and GDNF/Ret to promote neuroprotection and regeneration in DA neurons.
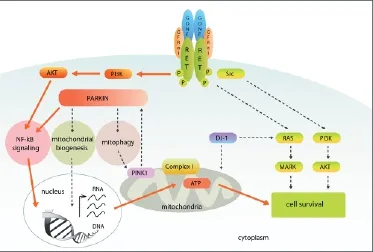
Figure 1 Converging signaling cascades of Ret and parkin to ensure mitochondrial integrity and SN DA neuron maintenance.
Synergistic effect of parkin and GDNF/Ret signaling on DA neuron protection and regeneration: Recently, we showed that the Ret receptor crosstalks with DJ-1 and parkin, two proteins encoded by genes found to be mutated in some familiar forms of PD (Aron et al., 2010; Meka et al., 2015). Single DJ-1 and parkin knockout mice showed no PD-related phenotype but in combination with Ret loss we could observe these proteins’ neuroprotective function. DJ-1/Ret and parkin/Ret double defi cient mice showed a more severe DA neuron degeneration phenotype compared to Ret-defi cient mice alone. While additional DJ-1 loss in Ret-defi cient mice reduced further the number of SNpc DA neurons but had no eff ect on the DA innervation of the striatum, additional parkin loss in Ret-defi cient mice enhanced the degeneration of substantia nigra DA neurons and the DA innervation in the striatum (Aron et al., 2010; Meka et al., 2015). DJ-1 and Ret signaling seems to converge on the Ras/ MAPK pathway to trigger cell survival, while both parkin and Ret use the NF-κB pathway to stimulate mitochondrial integrity (Aron et al., 2010; Meka et al., 2015) (Figure 1). In addition, we showed that overexpression of parkin can prevent the degeneration of DA neurons in Ret-defi cient mice (Meka et al., 2015). Our results suggest that parkin and Ret, in synergy, target mitochondrial complex I activity and ATP production. Parkin and Ret are also essential insuring proper mitochondrial morphology in SH-SY5Y cells. Overexpression of one of these proteins can compensate for the absence of the other (Meka et al., 2015). Both proteins also seem to infl uence, via Akt, important cell survival signal pathways such as p53 and caspases (Aron and Klein, 2011; Winklhofer, 2014). While GDNF/Ret signaling mediates neuroprotection in toxin models of PD, parkin seems more effi cient in protecting cells against stress caused, for instance, by α-synuclein accumulation (Ulusoy and Kirik, 2008). This suggests that the signaling cascades of Ret and parkin are not 100% identical, but that there are some converging signaling pathways like the NF-κB/mitochondria pathway which are used by both proteins. Besides the above mentioned functions of parkin, which may allow parkin to enhance DA neuron regeneration, the crosstalk with GDNF/Ret opens up the possibility that parkin might also support axonal regeneration. Therefore, the combined approach of enhancing parkin and Ret signaling, for example by gene therapy, might be considered to protect the remaining DA neurons and perhaps even helps to stimulate regeneration of DA axons in PD patients. We are currently conducting experiments to characterize further aspects of the parkin and GDNF/Ret crosstalk and hope to shed light on parkin’s potential regenerative function.
I thank Julia Kuhl for help with the art work and together with Mahmoud Bassal for comments on the paper. We apologize to all authors whose work could not be cited here due to paper length restrictions. This study was partly supported by grants from the DFG (KR 3529/4-1 to ERK) and the town of Hamburg (Lexi to ERK).
Edgar R. Kramer*
Development and Maintenance of the Nervous System, Center for Molecular Neurobiology, University Medical Center
Hamburg-Eppendorf, Hamburg, Germany
*Correspondence to: Edgar R. Kramer, Ph.D., kramer@zmnh.uni-hamburg.de.
Accepted: 2015-07-15
orcid: 0000-0002-4882-4143 (Edgar R. Kramer)
Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383-394.
Airavaara M, Voutilainen MH, Wang Y, Hoffer B (2012) Neurorestoration. Parkinsonism Relat Disord Suppl 1:S143-146.
Aron L, Klein R (2011) Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci 34:88-100.
Aron L, Klein P, Pham TT, Kramer ER, Wurst W, Klein R (2010) Pro-survival role for Parkinson’s associated gene DJ-1 revealed in trophically impaired dopaminergic neurons. PLoS Biol 8:e1000349.
Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Bjorklund A (2012) alpha-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci Transl Med 4:163ra156.
Kopra J, Vilenius C, Grealish S, Harma MA, Varendi K, Lindholm J, Castren E, Voikar V, Bjorklund A, Piepponen TP, Saarma M, Andressoo JO (2015) GDNF is not required for catecholaminergic neuron survival in vivo. Nat Neurosci 18:319-322.
Kowsky S, Poppelmeyer C, Kramer ER, Falkenburger BH, Kruse A, Klein R, Schulz JB (2007) RET signaling does not modulate MPTP toxicity but is required for regeneration of dopaminergic axon terminals. Proc Natl Acad Sci U S A 104:20049-20054.
Kramer ER, Aron L, Ramakers GM, Seitz S, Zhuang X, Beyer K, Smidt MP, Klein R (2007) Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol 5:e39.
Meka DP, Muller-Rischart AK, Nidadavolu P, Mohammadi B, Motori E, Ponna SK, Aboutalebi H, Bassal M, Annamneedi A, Finckh B, Miesbauer M, Rotermund N, Lohr C, Tatzelt J, Winklhofer KF, Kramer ER (2015) Parkin cooperates with GDNF/RET signaling to prevent dopaminergic neuron degeneration. J Clin Invest 125:1873-1885.
Paratcha G, Ledda F (2008) GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci 31:384-391.
Ulusoy A, Kirik D (2008) Can overexpression of parkin provide a novel strategy for neuroprotection in Parkinson’s disease? Exp Neurol 212:258-260.
Winklhofer KF (2014) Parkin and mitochondrial quality control: toward assembling the puzzle. Trends Cell Biol 24:332-341.
10.4103/1673-5374.165295 http://www.nrronline.org/
Kramer ER (2015) The neuroprotective and regenerative potential of parkin and GDNF/Ret signaling in the midbrain dopaminergic system. Neural Regen Res 10(11):1752-1753.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
- Studying neurological disorders using induced pluripotent stem cells and optogenetics
- Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces

