Hydrodynamics and Mass Transfer of Oily Micro-emulsions in An External Loop Airlift Reactor
Mona Ebrahimi Fakhari, Mostafa Keshavarz Moravejiand Reza Davarnejad
1Department of Chemical Engineering, Faculty of Engineering, Arak University, Arak 38156-8-8349, Iran
2Department of Chemical Engineering, Amirkabir University of Technology (Tehran Polytechnic), 424 Hafez Avenue, Tehran 15875-4413, Iran
Hydrodynamics and Mass Transfer of Oily Micro-emulsions in An External Loop Airlift Reactor
Mona Ebrahimi Fakhari1, Mostafa Keshavarz Moraveji2,*and Reza Davarnejad1
1Department of Chemical Engineering, Faculty of Engineering, Arak University, Arak 38156-8-8349, Iran
2Department of Chemical Engineering, Amirkabir University of Technology (Tehran Polytechnic), 424 Hafez Avenue, Tehran 15875-4413, Iran
This study reports an experimental investigation on hydrodynamics and mass transfer characteristics in a 15.6×10?3m3external loop airlift reactor for oil-in-water micro-emulsions with oil to water volume ratio (φ) ranging from 3% to 7% (by volume). For comparative purposes, experiments were also carried out with water. Increase in φ of micro-emulsion systems results in an increment in the gas holdup and a decrease in the volumetric gas-liquid oxygen transfer coefficient and liquid circulation velocity, attributed to the escalation in the viscosity of micro-emulsions. The gas holdup and volumetric mass transfer coefficient for micro-emulsion systems are significantly higher than that of water system. Two correlations are developed to predict the gas holdup and oxygen transfer coefficient.
external loop airlift reactor, hydrodynamics, micro-emulsions, oxygen transfer
1 INTRODUCTION
External loop airlift reactors (ELALRs) have emerged as one of the most promising devices in bioprocesses, wastewater treatment, and chemical industry due to their simple construction without internals or moving parts, well-defined flow pattern, low shear rate, low power consumption, good heat and mass transfer capacity, and good mixing characteristics [1-5].
The density difference of fluid in riser and down-comer sections of bioreactor is the driving force for circulation of liquid in an ELALR. The circulation creates good mixing in all phases and achieves good mass transfer. In addition, the external loop facilitates temperature control in the reactor [3, 6].
Oil in water (O/W) emulsion is such a waste by-product of many industries especially in chemical, petrochemical and oil refinery factories. Petroleum refineries generate large amounts of wastewater with high concentrations of hazardous contaminants. Because of the toxic nature and important effects of different kinds of oily wastewater on the surrounding environment (soil, water), it is necessary to treat the wastewater before discharging to the environment. Airlift reactor is a useful equipment for treating wastewaters.
Boltes et al. [7] studied the absorption of oxygen in water-dodecane emulsions in a bubble column bioreactor with internal recirculation loop. The volumetric mass transfer coefficients for oxygen (kLa) at various gas flow rates were determined in several organic fractions (from 0 to 100%).
Mehrnia et al. [8] measured volumetric oxygen transfer coefficients in an airlift reactor using water, kerosene, diesel and their emulsions in water (waterto-oil phase volume ratio in the range of 10%-30%). The values of kLa increased with gas velocity. The kLa values obtained for all the organic liquids were higher than that of water [8].
Moraveji et al. [9] investigated the effects of operating conditions and liquid properties on the hydrodynamic and mass transfer coefficient in an internal airlift reactor with oil-in-water micro-emulsions. The gas holdup increased and liquid circulation velocity (UL) and oxygen transfer coefficient decreased with the increase of the ratio of oil in water.
Several researches were carried out on the cross-sectional area ratio of down-comer (Ad) to riser (Ar) [2, 10], reactor height [3, 11], gas-liquid separator configuration [12], and distributor type and its location [13] in the ELALRs.
Kawase et al. [14] measured the mixing time (tm), circulation time and down-comer linear liquid velocity for two ELALRs (with Ad/Arratio of 0.204 and 0.458). The mixing time decreased with increasing superficial gas velocity (UG) and showed a constant trend beyond 2 cm·s?1. The mixing time also increased with increasing Ad/Arratio.
Choi considered the effects of horizontal connection length (0.1 m≤Lc≤0.5 m), cross-sectional area ratio of down-comer to riser (0.11≤Ad/Ar≤0.53) and superficial gas velocity (2 cm·s?1≤UG≤18 cm·s?1) on the gas holdup in the riser (εr) and down-comer (εd), liquid circulation velocity, mixing time, and overall volumetric mass transfer coefficient in an ELALR without an extension tube above the down-comer [15]. They found that the gas holdup and overall volumetric mass transfer coefficient in the riser and down-comer decreased with increasing horizontal connection length and increased with superficial gas velocity, whereas the circulation liquid velocity and mixing time showed various trends according to Ad/Arvalues.
Yazdian et al. [16] used an external airlift loop bioreactor for production of biomass from natural gas.The effects of cross-sectional area ratio of riser to down-comer, volume of gas-liquid separator, superficial gas velocity, and physical properties of gases and their mixtures on mixing time, gas holdup, and volumetric gas liquid mass transfer coefficients were investigated. Ar/Adhad remarkable effects on gas holdup and kLa due to its influence on mixing time. Kinematic viscosity (υ) showed its significant effect on mixing time, gas holdup and kLa for different gases (mixing time changes directly while gas holdup and kLa change indirectly).
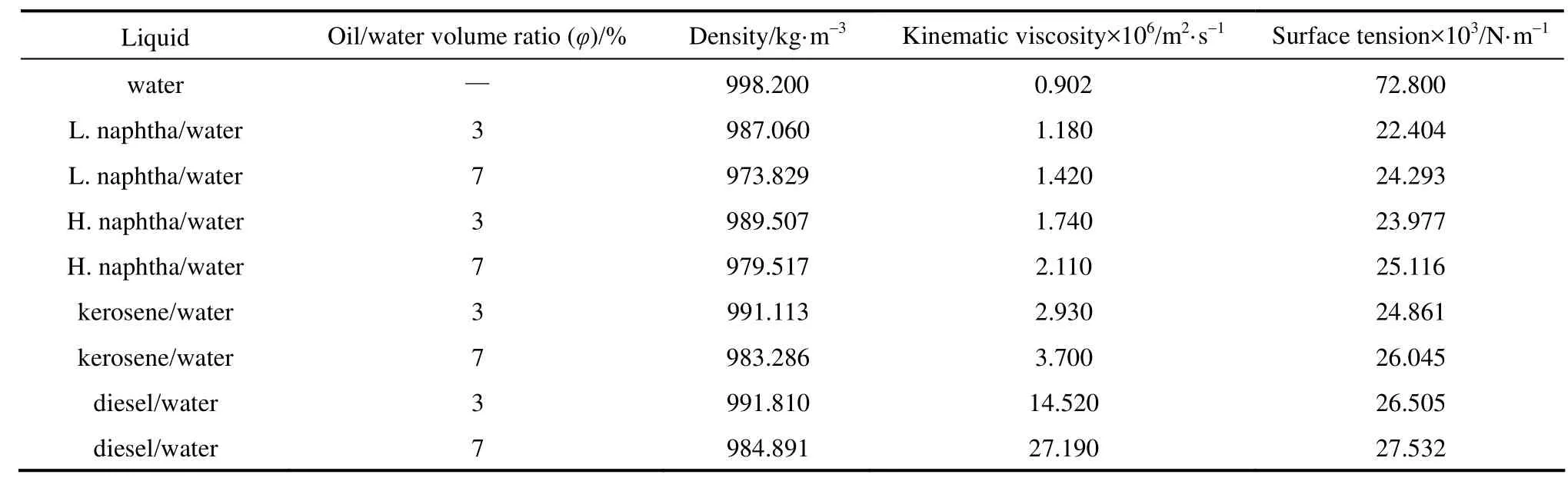
Table 1 Properties of liquids at 25 °C
Rujiruttanakul and Pavasant [17] examined various types of external loop airlift contactors for their hydrodynamic and mass transfer behavior. The investigation covered a variety of design parameters including the length of connection tubes, height of riser and down-comer, and airlift configurations while maintaining the cross-sectional area ratio between down-comer and riser constant at 0.269. The results demonstrated that the behavior of the external loop airlift could be modified by adjusting the design and operating variables. In general, a faster liquid velocity led to lower gas holdup and gas-liquid mass transfer rate. Increasing the length of connection tubes and height of riser and down-comer seemed to increase liquid velocity while decreasing the overall gas holdup (ε) and the overall volumetric mass transfer coefficient. Empirical correlations for the estimation of the system behavior were also formulated.
Hamood-ur-Rehman et al. [18] investigated the mixing characteristics in a packed bed external loop airlift bioreactor using tomography images. This bioreactor was characterized by an internal gas re-distributor between two packing beds in the riser with working fluid re-circulating through an external down-comer. The tomography images were employed to examine the effects of packing, gas re-distributor, sparger configuration, gas flow rate, and liquid height on the mixing time and superficial liquid velocity. Results showed that the mixing time in the bioreactor decreased with the increase of superficial gas velocity in the riser. At a constant superficial gas velocity in the riser, the mixing time increased and the superficial liquid velocity decreased when a cross shaped sparger was replaced by a circular sparger with the same diameter, and the same number and size of holes. The mixing time increased and the superficial liquid velocity decreased by reducing the liquid height in the bioreactor, in addition to installing the internal gas re-distributor and packing in the riser. The internal gas re-distributor and packing reduced the cross-sectional area available for flow and increased the resistance in the liquid flow path.
Some investigations were carried out on the hydrodynamics and mass transfer of oily micro-emulsions in the internal loop airlift reactors [8, 9] and of various chemicals such as water [17, 18] and aliphatic alcohols [19] in the ELALR. In this study, the hydrodynamics and mass transfer of oily micro-emulsions in ELALRs are investigated with various O/W micro-emulsion systems containing kerosene, heavy naphtha, light naphtha and diesel with concentrations of 3% and 7% (by volume). Empirical correlations are developed to predict the overall gas holdup and volumetric oxygen transfer coefficient.
2 EXPERIMENTAL
2.1 Materials
Various petroleum fractions containing kerosene, heavy naphtha, light naphtha and diesel were purchased from Shazand Oil Refinery Company (Arak, Iran) and their solutions with different concentrations [3% and 7% (by volume)] were locally prepared. Micro-emulsions were prepared from tap water and petroleum fractions. Nonil Phenol [(NF-60) with purity of 99.5% purchased from Isfahan Copolymer Company] was added as emulsifier. The liquid properties are summarized in Table 1.
2.2 Experimental set-up
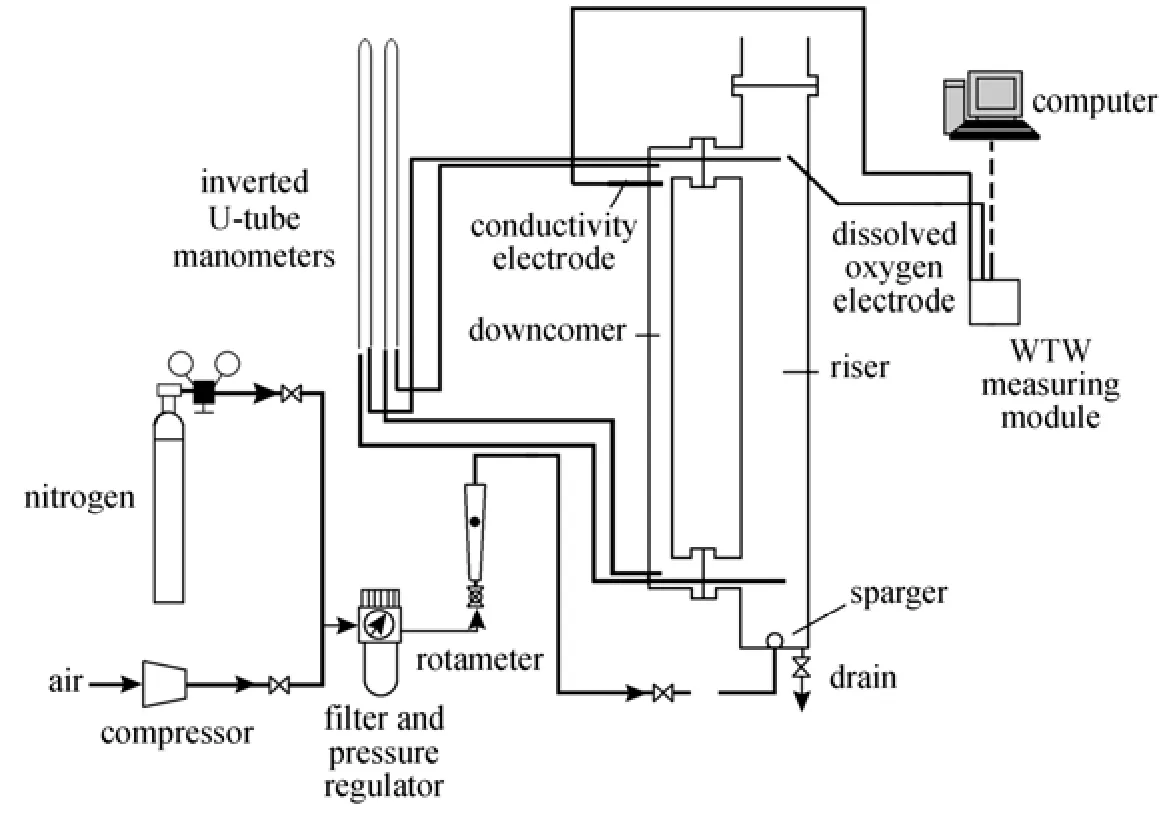
Figure 1 The schematic of the experimental setup
The schematic representation of the experimental setup is shown in Fig. 1. The external loop airlift reactor consists of two vertical pipes (a riser and a downcomer), a top connection pipe and a bottom connection pipe. The top section above the top horizontal connection acts as a gas-liquid separator. The airlift reactor is made of Plexiglas with a working volume of 0.0156 m3. Major dimensions of the reactor are given in Table 2. Air as the gas phase was injected into the riser through the gas sparger. The volumetric air flow rate in the riser zone was controlled by a regulating valve and a calibrated rotameter. The superficial gas velocity varied from 0.02 cm·s?1to 1.0 cm·s?1. The liquids employed were water and different O/W micro-emulsion systems containing kerosene, heavy naphtha, light naphtha and diesel as oil based with concentration of 3% and 7% (by volume).
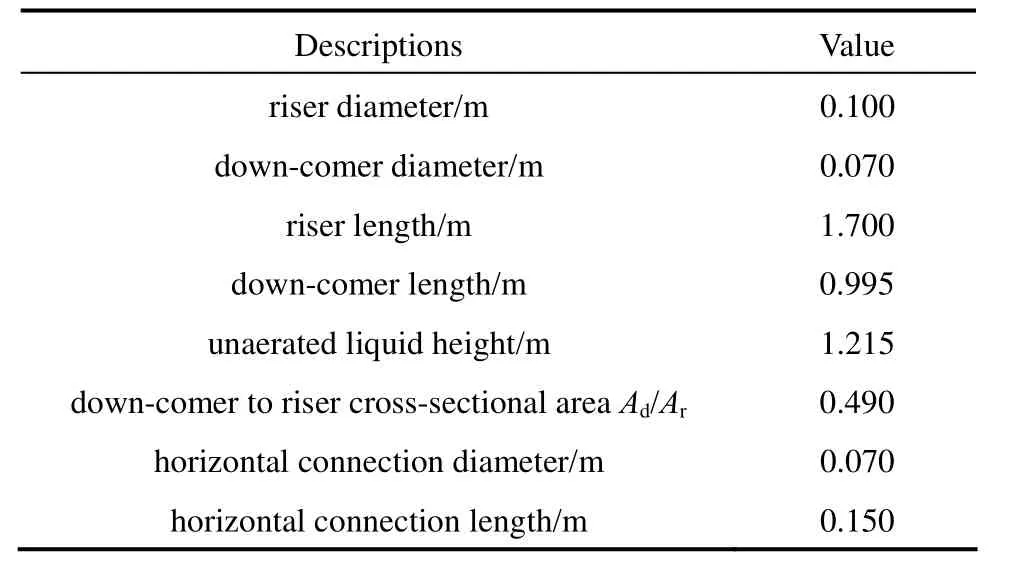
Table 2 Dimensions of the external loop airlift reactor
The inverted U-tube manometers were used for gas holdup measurement. A conductivity electrode (Model 740i, WTW, Germany) was used for liquid circulation velocity and mixing time measurements and a dissolved oxygen electrode (WTWCellox325) was used for oxygen concentration measurement in the liquid bulk. All experiments were carried out at ambient conditions [atmospheric pressure and (25 ± 0.5) °C].
2.3 Measurement methods
Volume expansion method was used to measure the overall gas holdup in the airlift bioreactor. The gas holdup in the riser (εr) and the down-comer zones (εd) were measured using the manometric method [20] with inverted U-tube manometers. These measurements were done after reaching the hydrodynamic steady state when no change was observed in the air flow rate.
The liquid circulation velocity in the reactor was determined by the classical tracer response technique based on very fast conductivity measurements [20]. The tracer (25 cm3, 1.5 mol·L?1NaCl) was instantaneously injected at the top of the down-comer zone, and the conductivity was monitored using the probe located in the down-comer zone. The sinusoidal conductivity response typically showed three to four peaks. The time difference between two adjacent peaks expresses the time required for one complete circulation of fluid in the airlift loop. Mixing time was estimated from the time interval between the first peak of the tracer signal and the point on χ-axis where the conductivity had 95 % of its final stable value.
The overall volumetric gas-liquid mass transfer coefficient was determined by the dynamic gassing-in method [21]. The dissolved oxygen concentration in the liquid phase was firstly reduced to nearly zero by nitrogen sparging. The nitrogen flow was stopped and all gas bubbles were allowed to escape from the liquid. The reactor was then sparged with air at a superficial velocity. During air sparging, the dissolved oxygen concentration was recorded until the liquid phase was almost saturated by oxygen. The overall volumetric oxygen mass transfer coefficient was calculated using the following equation, which is valid for large response time [22].
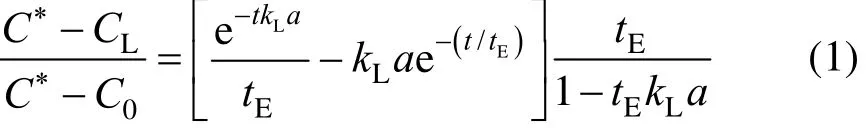
where tEis the electrode response time, C?is the saturation concentration of dissolved oxygen at operating temperature, and CLis the instantaneousconcentration at time t.
If the electrode time is so small (it occurs rarely), Eq. (1) is changed to
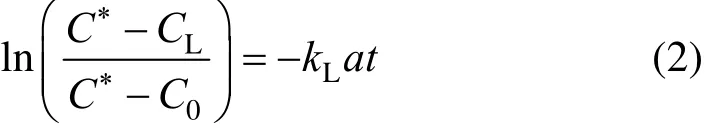
The slope of a plot of the left-hand side of Eq. (2) versus time provides the value of kLa.
3 RESULTS AND DISCUSSION
3.1 Gas holdup
Figure 2 shows the overall gas holdup data inside the ELALR with four O/W micro-emulsions versus the superficial gas velocity in the riser. These data are compared with those for pure water. The overall gas holdup for these systems increases with the increase of superficial gas velocity in the riser. The increase rate of gas holdup for micro-emulsion systems is slightly higher than that of pure water.
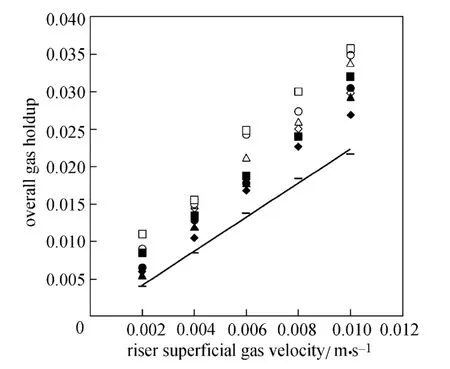
Figure 2 Overall gas holdup versus superficial gas velocity of riser
The results also show that the overall gas holdup for the oil-in-water micro-emulsion systems is higher than that of pure water system significantly. This difference can generally be attributed to much lower surface tension (σ) and higher coalescence hindering tendency of micro-emulsions with respect to water. Smaller bubbles are generated in the reactor filled with micro-emulsions and gas holdup increases.
Furthermore, viscosity affects the gas holdup by increasing drag force and reducing bubble rising velocity. In the current study, this positive effect of viscosity on gas holdup is stronger than its negative effect, due to the production of larger bubbles and enhancement of buoyancy force. In some solutions with high densities (ρ), the buoyancy force as well as increased bubbles made gas holdup decrease [9]. Shariati et al. [23] showed that gas holdup in the Isomax diesel and in the water in diesel micro-emulsion systems (with υ≤46.142×10?6m2·s?1) was significantly higher than that of water system, although McManamey and Wase [24] did not found change on the total gas holdup inside an external loop airlift bioreactor by adding glucose to water.
Figure 2 also shows that an increment in φ values increases gas holdup, similar to that observed by Moraveji et al. in an internal airlift reactor [9].
According to the data from the experiment in the ELALR for various O/W micro-emulsions, the gas holdup is correlated using dimensionless numbers

where Bo, Ga and Fr are Bond number, Galilei number and Froude number, respectively, the bubble diameters (db) range is from 2.20 to 2.40 mm.
Equation (3) correlates 97% of the data with about 16% error. Fig. 3 shows a comparison between experimental data and calculated results for the gas holdup. Other correlations were obtained from literature [25]. Akita and Yoshida’s equation [26] showed better agreement with the experimental data.
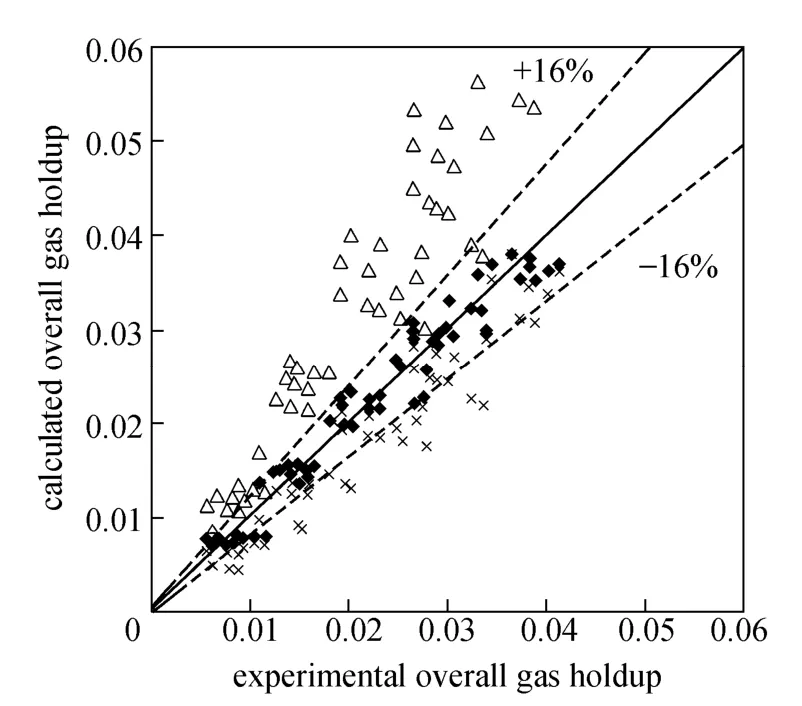
Figure 3 Comparison of experimental gas holdup and calculated results
Figures 4 and 5 show the gas holdup versus UGfor pure water and O/W micro-emulsions in the riser and down-comer, respectively. The volume fraction of gas in the riser increases with the gas velocity. Since more bubbles enter the down-comer, the gas holdup in the riser and down-comer increases. The trend is sharper for pure water in the riser in comparison with downcomer, while the trend is reverse for micro-emulsions systems.
3.2 Liquid circulation velocity
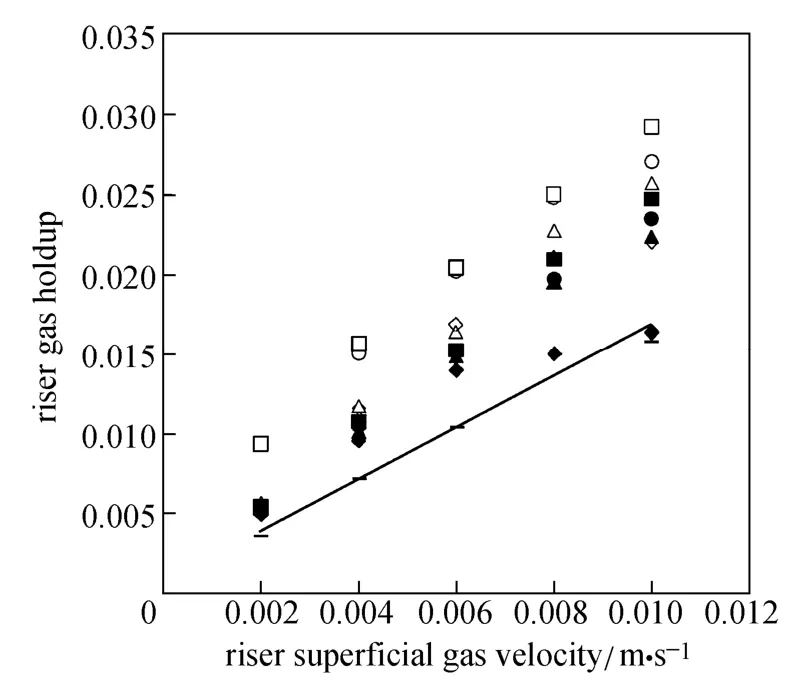
Figure 4 Gas holdup versus UGin the riser
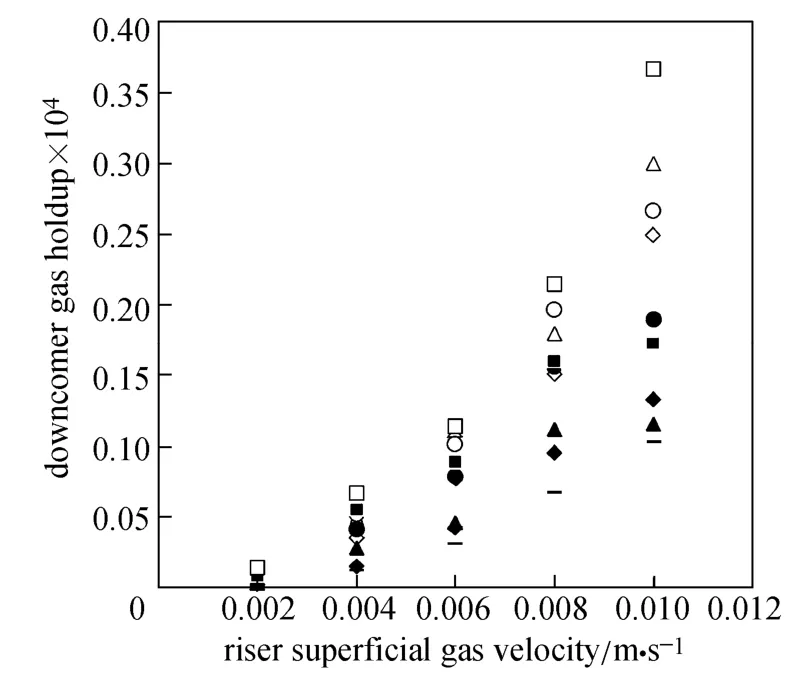
Figure 5 Gas holdup versus UGin the down-comer
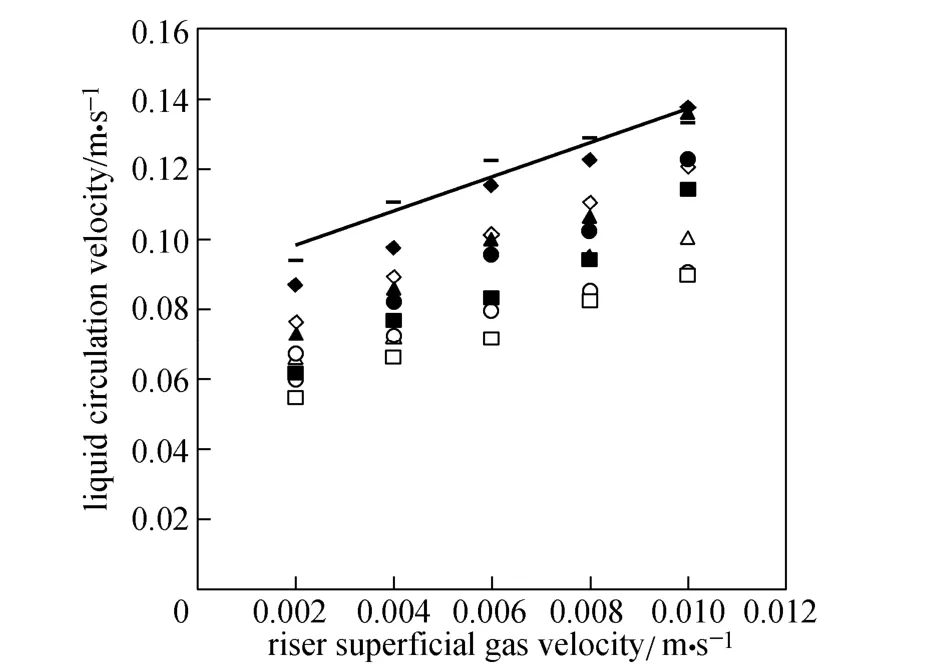
Figure 6 Liquid circulation velocity versus riser superficial gas velocity
Figure 6 shows typical experimental data for liquid circulation velocity versus superficial gas velocity of riser for various micro-emulsions in this study. In O/W micro-emulsion systems, smaller surface tension and bubble diameter lead to higher gas holdup, especially in the down-comer as explained earlier. The difference in gas holdup between riser and down-comer decreases and the driving force for liquid circulation decreases. Therefore, in micro-emulsions liquid circulation velocity is less than that in water. As O/W ratio increases, the liquid circulation velocity decreases as well.
3.3 Mixing time
Figure 7 shows the variation of mixing time versus superficial gas velocity for different ratios of O/W micro-emulsions (3% and 7%) and pure water. The mixing time decreases with increasing superficial gas velocity. Gavrilescu and Tudose [27] have performed extensive experimental studies on mixing and axial dispersion behavior of the liquid phase in two pilot scales of ELALR with different Ad/Arratios (0.040 and 0.1225). They found that mixing time decreased with increasing UG(in the range of 1-12 cm·s?1) and increased with Ad/Arratio.
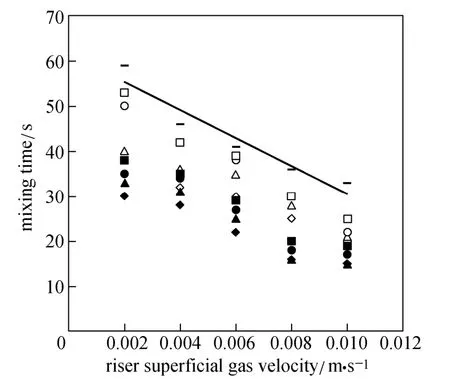
Figure 7 Mixing time versus riser superficial gas velocity
The mixing time shows an opposite trend in comparison with the liquid circulation velocity. Water has the longest mixing time in comparison with the micro-emulsions, since lower surface tension of micro-emulsions reduces bubble sizes. Circulation of these bubbles inside the reactor assists the mixing process.
3.4 Overall volumetric oxygen transfer coefficient
Figure 8 show the overall volumetric oxygen transfer coefficient (kLa) in the ELALR versus superficial gas velocity in the riser. Similar to the gas holdup, kLa values for all micro-emulsions are significantly higher than those for the water.
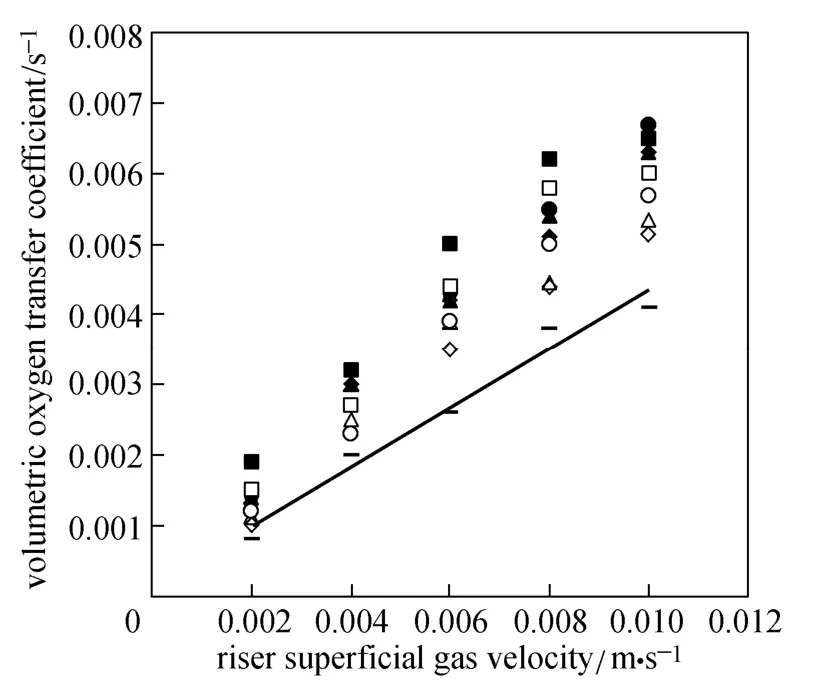
Figure 8 Overall volumetric oxygen transfer coefficient versus riser superficial gas velocity
Since the surface tension of water is higher than (around three times) that of the micro-emulsions, bubble size decreases and gas-liquid interfacial area (a) increases in the micro-emulsions, improving the mass transfer. Viscosity is a bulk liquid property that plays a major role in the overall mass transfer. Higher liquid viscosity increases bubble coalescence rate, so the gas-liquid interfacial area decreases. Higher liquid viscosity increases the thickness of liquid boundary layer by overall bubbles or lower solute diffusivity [28]. Thus liquid film mass transfer coefficient (kL) decreases [29]. Buoyancy force increases with density. It assists bubbles to leave the reactor. In the liquid with higher density, bubbles rise easily. That is, by increasing the density of liquid, the time for mass transfer between two phases is not enough, so kLa values decrease with increasing micro-emulsion concentration.
In our study in the ELALR, kLa values are in the range of 0.0008-0.0041, 0.001-0.00515, 0.0011-0.00535, 0.0012-0.0057 and 0.0015-0.006 s?1for pure water, diesel, kerosene, heavy naphtha and light naphtha, respectively , while they were in the range of 0.0031-0.012, 0.0037-0.0121, 0.0048-0.0149, 0.0038-0.0158 and 0.0043-0.0166 s?1, respectively, in an internal loop airlift reactor (under the same conditions) [9]. The deviation between the ELALR data and internal loop airlift reactor data is due to separating gas and liquid at the head zone of ELALR.
Furthermore, Mehrnia et al. found that the increment of water to oil volume ratio (from 10% to 30%) in petroleum solutions increased solution viscosity and decreased kLa values at a constant superficial gas velocity inside a draft tube airlift bioreactor [8].
By using the current experimental data, the volumetric mass transfer coefficient for micro-emulsions is correlated using dimensionless numbers
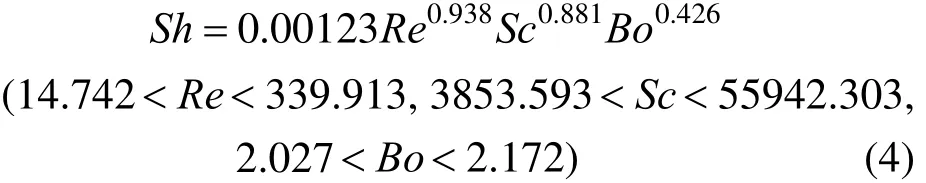
where Sh, Re and Sc are Sherwood number, Reynolds number and Schmidt number, respectively. The determination coefficient (R2) is around 0.96.
Figure 9 shows the calculated data with various equations in literature [25] and our correlation versus the experimental data for the micro-emulsions systems. Asgharpour et al.’s equation [30] shows better agreement with the experimental data.
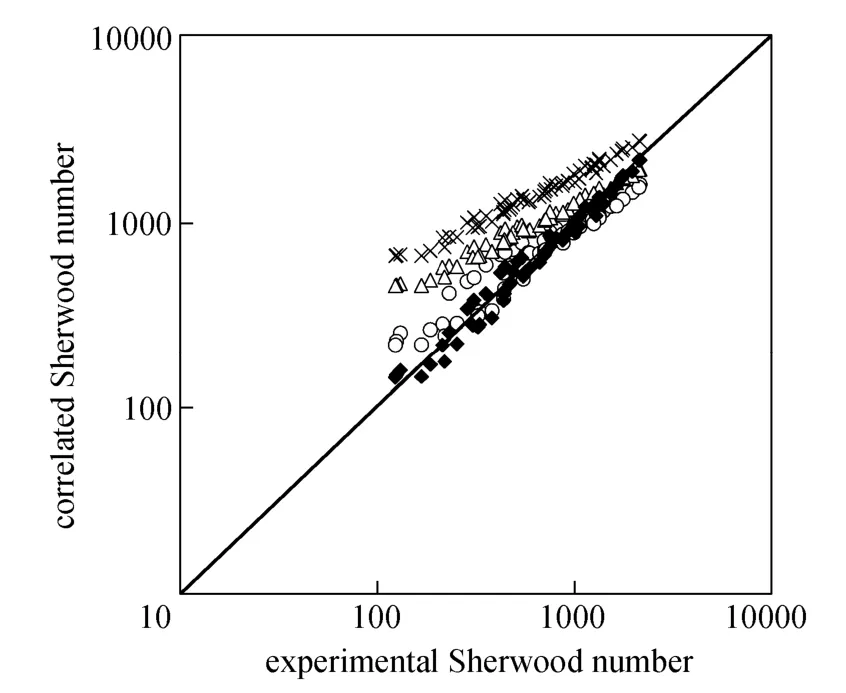
Figure 9 Comparison of calculated data from correlations and experimental data
4 CONCLUSIONS
This study reported the experimental investigation on hydrodynamics and mass transfer characteristics of four oil-in-water micro-emulsions [containing light naphtha, heavy naphtha, kerosene and diesel with concentration of 3% and 7% (by volume)] in an external loop airlift reactor. Comparison with pure water, the volumetric mass transfer coefficient and gas holdup in micro-emulsion systems are higher, while mixing time and liquid circulation velocities are lower. Furthermore, gas holdup increases with increasing concentration of oil in the micro-emulsions. The two correlations for overall gas holdup and volumetric mass transfer coefficient are in satisfactory agreement with experimental data.
NOMENCLATURE
BoBond number
CLconcentration of dissolved oxygen, kg·m?3
C0initial concentration of dissolved oxygen, kg·m?3
C?saturation concentration of dissolved oxygen, kg·m?3
D gas diffusivity, m2·s?1
dbbubble diameter, m
FrFroud number
Adcross-sectional area of down-comer, m2
Arcross-sectional area of riser, m2
a gas-liquid interfacial area per unit liquid volume, m2·m?3
GaGalilie number
g acceleration due to gravity, m·s
kLliquid film mass transfer coefficient, m·s?1
kLa overall volumetric gas-liquid mass transfer coefficient, s?1
Lchorizontal connection length, m
ReReynolds number
ShSherwood number
Sc Schmit number (/ )Dμρ
t time, s
tEelectrode response time, s
tmmixing time, s
UGsuperficial aeration velocity in the riser zone, m·s?1
ULliquid circulation velocity, m·s?1
ε overall gas holdup
εdgas holdup in down-comer
εrgas holdup in riser
μ viscosity, Pa·s
ρ density, kg·m?3
σ surface tension, N·m?1
υ kinematic viscosity, m2·s?1
φ oil to water volume ratio
REFERENCES
1 Merchuk, J.C., Stein, Y., “Local hold-up and liquid velocity in airlift reactors”, AIChE J., 27, 377-388 (1981).
2 Choi, K.H., Lee, W.K., “Circulation liquid velocity, gas hold-up and volumetric oxygen transfer coefficient in external-loop airlift reactors”, J. Chem. Technol. Biotechnol., 56, 51-58 (1993).
3 Bentifraouine, C., Xuereb, C., Riba, J.P., “An experimental study of the hydrodynamic characteristics of external loop airlift contactors”, J. Chem. Technol. Biotechnol., 69, 345-349 (1997).
4 Ferreira, A., Ferreira, C., Teixeira, J.A., Rocha, F., “Temperature and solid properties effects on gas-liquid mass transfer”, Chem. Eng. J., 162, 743-752 (2010).
5 Yang, J.H., Yang, J., Kim, H.J., Chun, D.H., Lee, H., Jung, H., “Two regime transitions to pseudo-homogeneous and heterogeneous bubble flow for various liquid viscosities”, Chem. Eng. Process., 49, 1044-1050 (2010).
6 Luo, L.J., Liu, F.N., Xu, Y.Y., Yuan, J.Q., “Hydrodynamics and mass transfer characteristics in an internal loop airlift reactor with different spargers”, Chem. Eng. J., 175, 494-504 (2011).
7 Boltes, K., Caro, A., Leton, P., Rodriguez, A., Garcia-Calvo, E.,“Gas-liquid mass transfer in oil-water emulsions with an airlift bio-reactor”, Chem. Eng. Process., 47, 2408-2412 (2008).
8 Mehrnia, M.R., Towfighi, J., Bonakdarpour, B., Akbarnejad, M.M.,“Gas holdup and oxygen transfer in a draft-tube airlift bioreactor with petroleum based liquids”, Biochem. Eng. J., 22, 105-110 (2005).
9 Moraveji, M.K., Sajjadi, B., Davarnejad, R., “The influence of oil-water ratio on the operation of an airlift reactor containing petroleum-based micro-emulsion”, Pet. Sci. Technol., 32 (5), 514-520 (2014).
10 Young, M.A., Carbonell, R.G., Ollis, D.F., “Airlift bioreactors: Analysis of local two-phase hydrodynamics”, AIChE J., 37, 403-428 (1991).
11 Russell, A.B., Thomas, C.R., Lilly, M.D., “The influence of vessel height and top-section size on the hydrodynamic characteristics of airlift fermentors”, Biotechnol. Bioeng., 43, 69-76 (1994).
12 Siegel, M.H., Merchuk, J.C., “Hydrodynamics in rectangular airlift reactors: scale-up and the influence of gas-liquid separator design”, Can. J. Chem. Eng., 69, 465-473 (1991).
13 Becker, S., Sokolichin, A., Eigenberger, G., “Gas-liquid flow in bubble column and loop reactors. Part II. Comparison of detailed experiments and flow simulations”, Chem. Eng. Sci., 49, 5747-5762 (1994).
14 Kawase, Y., Omori, N., Tsujimura, M., “Liquid-phase mixing in external-loop airlift bioreactor “, J. Chem. Technol. Biotechnol., 61, 49-55 (1994).
15 Choi, K.H., “Hydrodynamic and mass transfer characteristics of external-loop airlift reactors without an extension tube above the downcomer”, Korean J. Chem. Eng., 18, 240-246 (2001).
16 Yazdian, F., Shojaosadati, S.A., Nosrati, M., Pesaran Hajiabbas, M., Vasheghani-Farahani, E., “Investigation of gas properties, design, and operational parameters on hydrodynamic characteristics, mass transfer, and biomass production from natural gas in an external airlift loop bioreactor”, Chem. Eng. Sci., 64, 2455-2465 (2009).
17 Rujiruttanakul, Y., Pavasant, P., “Influence of configuration on the performance of external loop airlift contactors”, Chem. Eng. Res. Des., 89, 2254-2261 (2011).
18 Hamood-ur-Rehman, M., Dahman, Y., Ein-Mozaffari, F., “Investigation of mixing characteristics in a packed-bed external loop airlift bioreactor using tomography images”, Chem. Eng. J., 213, 50-61 (2012).
19 Gharib, J., Moraveji, M.K., Davarnejad, R., Malool, M.E., “Hydrodynamics and mass transfer study of aliphatic alcohols in airlift reactors”, Chem. Eng. Res. Des., 91,925-932 (2013).
20 Chisti, M.Y., “New investigation of gas properties, design, and operational parameters on hydrodynamic characteristics, mass transfer, and biomass production from natural gas in an external airlift loop bioreactor”, Airlift Bioreactors, Elsevier Science Publishers, New York (1989).
21 Kalekar, M.S., Bhagwat, S.S., “Dynamic behavior of surfactants in solution”, J. Dispersion Sci. Technol., 27, 1027-1034 (2006).
22 Garcia-Ochoa, F., Gomez, E., “Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview”, Biotechnol. Adv., 27, 153-176 (2009).
23 Shariati, F.P., Bonakdarpour, B., Mehrnia, M.R., “Hydrodynamics and oxygen transfer behavior of water and diesel microemulsions in a draft tube airlift bioreactor”, Chem. Eng. Process., 46, 334-342 (2007).
24 McManamey, W.J., Wase, D.A.J., “The relationship between the volumetric mass transfer coefficient and gas holdup in airlift fermentors”, Biotechnol. Bioeng., 28, 1446-1448 (1986).
25 Sajjadi, B., Moraveji, M.K., Davarnejad, R., “Investigation of surfactant effect on the operational characteristics of a packed bed internal loop airlift reactor”, World Applied Sciences J., 11, 1004-1014 (2010).
26 Akita, K., Yoshida, F., “Bubble size interfacial area and liquid-phase mass transfer coefficient in bubble columns”, Ind. Eng. Chem. Proc. Des. Dev., 13, 84-91 (1974).
27 Gavrilescu, M., Tudose, R.Z., “Mixing studies in external-loop reactors”, Chem. Eng. J., 66, 97-104 (1997).
28 Wilke, C.R., Chang, P., “Correlation of diffusion coefficients in dilute solutions”, AIChE J., 1, 264-270 (1955.).
29 Kilonzo, P.M., Margaritis, A., “The effects of non-Newtonian fermentation broth viscosity and small bubble segregation on oxygen mass transfer in gas-lift bioreactors: A critical review”, Biochem. Eng. J., 17, 27-40 (2004).
30 Asgharpour, M., Mehrnia, M.R., Mostoufi, N., “Effect of surface contaminants on oxygen transfer in bubble column reactors”, Biochem. Eng. J., 49, 351-360 (2010).
FLUID DYNAMICS AND TRANSPORT PHENOMENA
Chinese Journal of Chemical Engineering, 22(3) 267—273 (2014)
10.1016/S1004-9541(14)60050-1
2012-12-07, accepted 2013-08-18.
*To whom correspondence should be addressed. E-mail: moraveji@aut.ac.ir
 Chinese Journal of Chemical Engineering2014年3期
Chinese Journal of Chemical Engineering2014年3期
- Chinese Journal of Chemical Engineering的其它文章
- A Bi-component Cu Catalyst for the Direct Synthesis of Methylchlorosilane from Silicon and Methyl Chloride
- Numerical Modeling and Analysis of Gas Entrainment for the Ventilated Cavity in Vertical Pipe*
- Effects of Shape and Quantity of Helical Baffle on the Shell-side Heat Transfer and Flow Performance of Heat Exchangers*
- A Contraction-expansion Helical Mixer in the Laminar Regime*
- Preparation and Characterization of Sodium Sulfate/Silica Composite as a Shape-stabilized Phase Change Material by Sol-gel Method*
- Determination of Transport Properties of Dilute Binary Mixtures Containing Carbon Dioxide through Isotropic Pair Potential Energies
