Therapeutic Effect and Safety of Ustekinumab for Plaque Psoriasis: A Meta-analysis
Yi Liu, Jian-ping Gong, and Wen-fang Li*
1Department of Dermatology, Luzhou Medical College, Luzhou, Sichuan 646000, China
2Department of Hepatibiliary Surgery, the Second Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China
PSORIASIS is a chronic inflammatory skin disease which is easy to relapse. Along with the deepening research on the pathology of psoriasis, it is recognized as a disease of polygenic inheritance under the background of autoimmune disorder, especially associating with T cell-mediated immune. In the pathogenic process, the initial T cells are activated to memory T cells, which entering into the circulation and migration to the skin, meanwhile, a variety of cytokines are secreted.1Interleukin 12/interleukin 23 (IL-12/IL-23) cytokines have been identified as critical molecules and mediators in psoriasis disease progression.2,3
Chronic plaque psoriasis is the most common type of psoriasis and characterized by redness, thickness, and scaling.4Ustekinumab is a humanized anti-IL-12/IL-23 monoclonal antibody which could specially bind with IL-12 and IL-23 p40 subunits and inhibit the biological activity of IL-12/IL-23. Many clinical trials reported that ustekinumab could effectively treat plaque psoriasis. The U.S. Food and Drug Administration, the European Medicines Agency and several regulatory agencies in other countries have approved ustekinumab for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for phototherapy or systemic therapy.5But the long-term effectiveness and safety of ustekinumab for the treatment of psoriasis need more research and analysis.
By way of collecting published clinical research results, and/or unpublished screening quality standard literature all over the world, more reliable conclusions could be gotten through system qualitative or quantitative analysis. Ustekinumab for the treatment of psoriasis demonstrated higher efficacy rates compared with traditional therapies.6But as for plaque psoriasis, the therapeutic efficacy and safety of ustekinumab are unclear yet. In this study, we collected the latest clinical trial data, and evaluated the efficacy and safety of ustekinumab in the therapy of plaque psoriasis, in order to supply more reliable and applicable results.
MATERIALS AND METHODS
Search strategy
We performed a computer or manual literature search in Cochrane Central Register of Controlled Trials, MEDLINE, and PubMed from inception up to November 2013 in order to collect clinical data of patients with plaque psoriasis undergoing ustekinumab therapy. The used key terms were “ustekinumab” or “plaque psoriasis” or “psoriasis” or “IL-12/IL-23” or “randomized controlled trial”.
Inclusion criteria
The observational studies selected into this meta-analysis had to conform the following standard: (1) research or report related the association between ustekinumab and therapy for plaque psoriasis; (2) studies were either cohort or case-control designs; (3) the diagnosis of plaque psoriatic patients reported in these studies was definite; (4) definition of ustekinumab therapeutic history must be clear; (5) the reported data were integrated and clear, such as the total sample, the number of cases, odds ratio (OR), and relative risk (RR); (6) if two or more studies were reported by the same authors in the same institution, either the study of higher quality or the most recent publication was included in the analysis.
Exclusion criteria
The exclusion criteria included: (1) pustular psoriasis or psoriasis guttata; (2) ustekinumab in combination with other drugs; (3) repetition, poor quality, and little information report; (4) there were no the information of original data; (5) case report or the small sample studies.
Quality assessment and data extraction
The methodological quality of the trails was assessed by the Jadad scale and high quality studies (Jadad score 5) were included in this study. Two independent reviewers extracted the data and then compared the results. They would discuss inconsistent results together and then made the decision. Relevant data were extracted from the text, including the type of research design, research publication time, study region, the overall sample size, the number of plaque psoriatic cases, OR, RR, and 95% confidence interval (CI). Psoriasis Area and Severity Index (PASI) was used to evaluate the severity of plaque psoriasis. PASI score range is from 0 (no disease) to 72 (maximal disease). A 75% reduction in the PASI score (PASI 75) is usually used to evaluate the therapeutic effect of plaque psoriasis.7-9
Statistical analysis
In this study, statistical analyses were conducted using Review Manager 5.1 software provided by the Cochrane Collaboration. Count data were presented as RR with 95% CI. Heterogeneity between studies was assessed using Chi-square (χ2) test with significance level set at P<0.1. The meta-analysis was done using the fixed or random- effect model. Statistical significance of the pooled studies was determined by Z test. P<0.05 was considered statistically significance. Funnel graph was used to analyze the publication bias.
RESULTS
Basic information included in these studies have no difference
Six randomized control trials with a 5 points Jadad scale score were included in this study.7,10-14There were 1012 cases in the treated group and 985 cases in the placebo group. There were no significant differences of the baseline comparison before treatment including number of cases, age, sex distribution, duration of psoriasis, average PASI score, proportion of psoriatic arthritis (P=0.528, 0.670, 0.283, 0.574, 0.117, 0.872 respectively, all P>0.05; Table 1).

Table 1. General information of the included studies
Ustekinumab has significantly therapeutic effect on plaque psoriasis
From the funnel plot, we found that there was no publication bias in the 6 randomized control trials (Fig. 1). A pooled analysis of the 6 trials7,10-14using a random-effect model (I2=57%, P=0.04) indicated that ustekinumab 45 mg group could get better therapeutic effect compared with the placebo group (P<0.00001, Fig. 2). The RR was 13.76 and 95% CI [8.37, 22.60]. A pooled analysis of the 4 trials7,10-12using a fixed model (I2=0%, P=0.74) indicated that ustekinumab 90 mg group could get obviously better therapeutic effect compared with the placebo group (P<0.00001, Fig. 3). The RR was 20.41 and 95% CI [13.98, 29.80]. The same 4 trials7,10-12using a fixed model (I2=11%, P=0.34) indicated that ustekinumab 90 mg group could get better therapeutic effect compared with ustekinumab 45 mg group (P=0.01, Fig. 4). The RR was 0.92 and 95% CI [0.86, 0.98].
Adverse effects
Adverse effects in the 6 trials were mentioned including headache, upper respiratory tract infection, and nasopharyngtis. The serious adverse effects included serious infection, cardiovascular events, and malignant tumors. There were no significant differences in the adverse effects of headache (P=0.17), upper respiratory tract infection (P=0.51), nasopharyngitis (P=0.19) between ustekinumab 45 mg group and the placebo group. But the infection in ustekinumab 45 mg group was significantly higher than the placebo group (P=0.02, Fig. 5). There were no significant differences in the adverse effects of headache (P=0.22), upper respiratory tract infection (P=0.60), nasopharyngitis (P=0.94), infection (P=0.68), serious infection (P=0.89), cardiovascular events (P=0.87), and malignant tumor (P=0.55) between ustekinumab 90 mg group and the placebo group (Fig. 6). There were no significant differences in the adverse effects of headache (P=0.89), upper respiratory tract infection (P=0.64), nasopharyngitis (P=0.68), infection (P=0.37), serious infection (P=0.15), cardiovascular events (P=0.48), and malignant tumor (P=0.69) between ustekinumab 45 mg group and 90 mg group (Fig. 7).
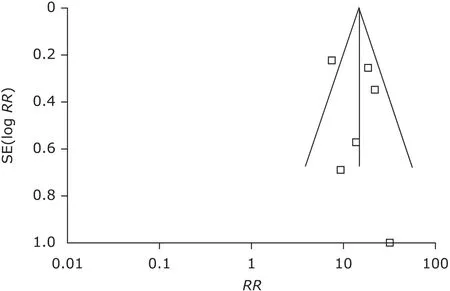
Figure 1. Funnel plot of publication bias. RR: relative risk; SE: standard error.
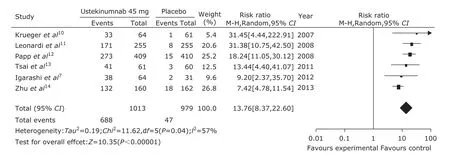
Figure 2. Forest plot of therapeutic effect comparing ustekinumab 45 mg group with the placebo group at 12th week. 95% CI: 95% confidence interval.

Figure 3. Forest plot of therapeutic effect comparing ustekinumab 90 mg group with the placebo group at 12th week.
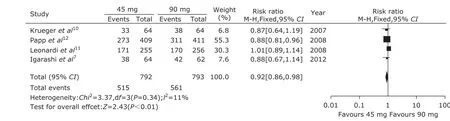
Figure 4. Forest plot of therapeutic effect comparing ustekinumab 45 mg group with 90 mg group at 12th week.
DISCUSSION
Psoriasis is a chronic, immune-mediated inflammatory disease, which is characterized by hyperplasia of the epidermis and infiltration of leukocytes into both the dermis and epidermis. It is estimated that approximately 2%-4% population suffer from psoriasis at present.8Plaque psoriasis is the most common form of psoriasis. There is no available cure method for psoriasis, and some medicines such as methotrexate, ring spore element A, local or system application of glucocorticoid might take effect. However, due to a lack of tissue specificity drug, the curative effect is not optimistic, and long-term use may have serious side effects or inconvenience.9IL-12 and IL-23 are known to play an important role in the pathogenesis of psoriasis. IL-12 and IL-23 are hetero dimers with a common p40 subunit, which binding with corresponding receptor results in activation of specific intracellular signaling cascades.
Ustekinumab is a novel human monoclonal antibody that binds to the shared p40 subunit of IL-12 and IL-23 and prevents the relapse of the cytokines, thus blocking the downstream signaling cascades.7,12-14Recently, many clinical trials reported that ustekinumab could effectively inhibit function of IL-12/IL-23, and get apparently ther- apeutic effect in moderate-to-severe plaque psoriasis.7,13In this meta research, we analyzed the patients reached PASI 75 at week 12 in all the six trials. Compared with the placebo group, there were significant therapeutic effects for plaque psoriasis in both ustekinumab 45 mg group and 90 mg group. Furthermore, the therapeutic effect in ustekinumab 90 mg group appeared more marked than 45 mg group. So, we concluded that ustekinumab was an effective therapeutic method for plaque psoriasis and the therapeutic effect was positively correlated with the drug dose.
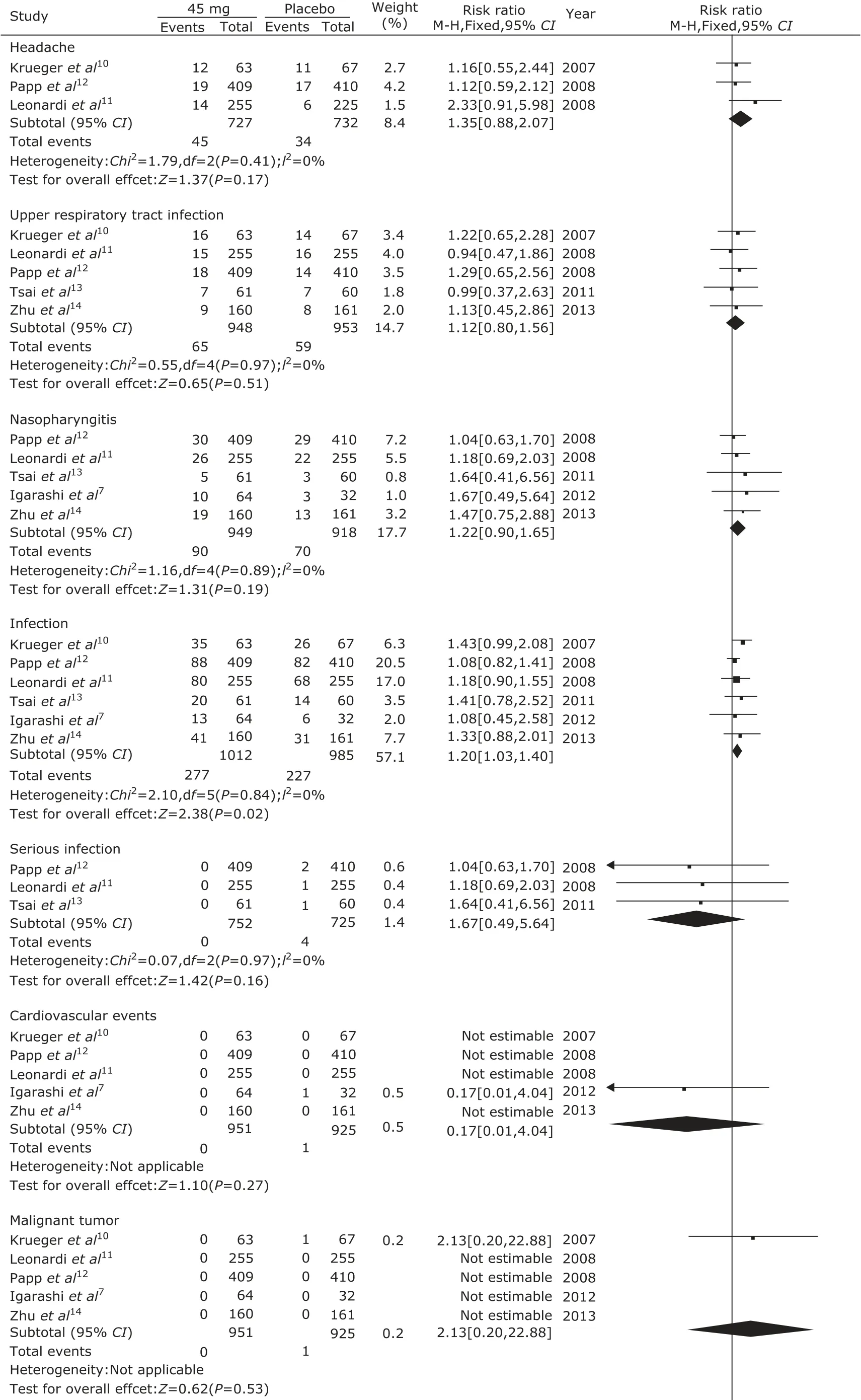
Figure 5. Meta-analysis of adverse effects between ustekinumab 45 mg group and the placebo group at 12th week.
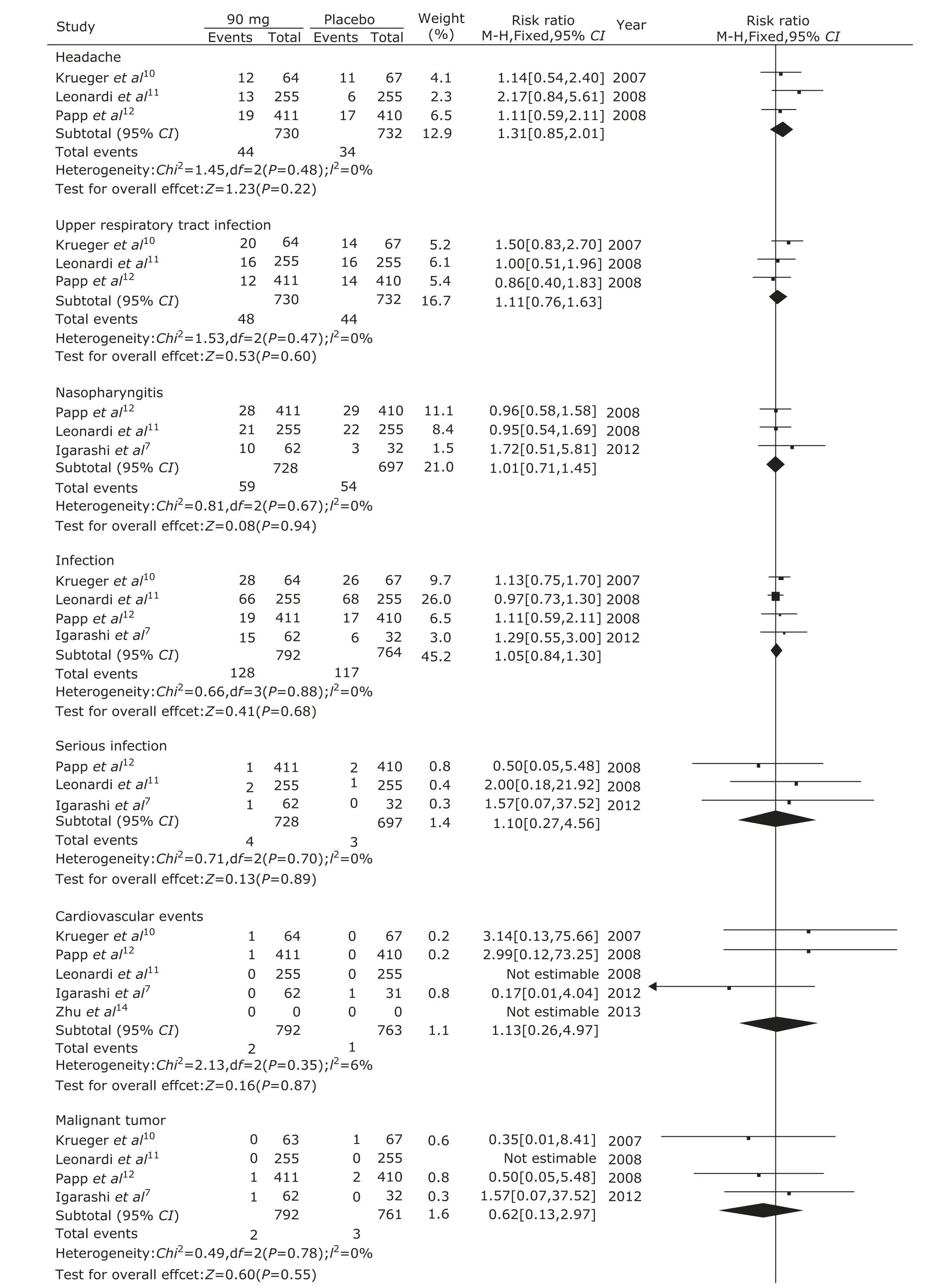
Figure 6. Meta-analysis of adverse effects between ustekinumab 90 mg group and the placebo group at 12th week.
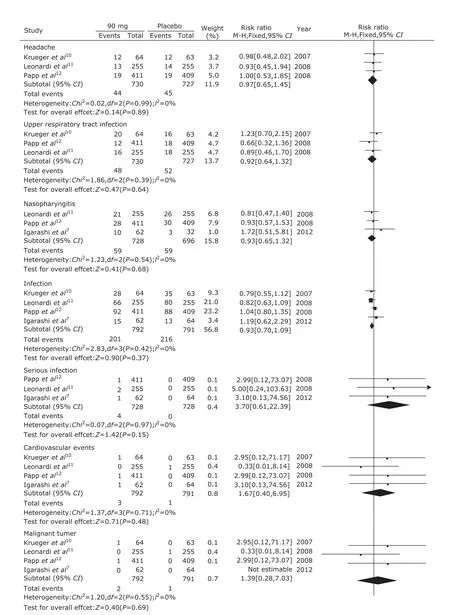
Figure 7. Meta-analysis of adverse effects between ustekinumab 45 mg group and 90 mg group at 12th week.
In addition, we further analyzed the adverse effects of ustekinumab. We found that there were no significant differences in common adverse effects in three groups, such as headache, upper respiratory infection, and naso- pharyngitis. However, the incidence of infection in ustekinumab 45 mg group was higher than the placebo group, while the difference did not appear between ustekinumab 90 mg group and the placebo group. And the same infection rate was also found between ustekinumab 45 mg group and 90 mg group. So, whether ustekinumab could increase the infection rate was still unclear. The same incidence of malignant tumor and cardiovascular events which usually regarded as seriously adverse events was also found in the ustekinumab and the placebo groups. We considered that longer-term follow-up was needed to observe occurrence of potential uncovered adverse reaction.
Because the included studies in this meta-analysis were less, and the usage of ustekinumab was consistent and the follow-up time was shorter, there was a certain bias. So the conclusion was further confirmed by more researches and longer time follow-up. And also in this study, the sustainability of curative effect was not clear. We thought that further attention would need more longer-term observation to verify the safety and efficacy of ustekinumab for the treatment of plaque psoriasis.
In conclusion, ustekinumab appears to be more effective and safe for plaque psoriasis. However, further analysis of longer-term safety is needed.
1. Caca Biljanovska N, V'lckova Laskoska M. Principles of biological therapy in psoriasis. Prilozi 2013; 34: 143-53.
2. Hueber AJ, McInnes IB. Immune regulation in psoriasis and psoriatic arthritis—recent developments. Immunol Lett 2007; 114: 59-65.
3. Wada Y, Cardinale I, Khatcherian A, et al. Apilimod inhibits the production of IL-12 and IL-23 and reduces dendritic cell infiltration in psoriasis. PLoS One 2012; 7: e35069.
4. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: An abridged Cochrane Systematic Review. J Am Acad Dermatol 2013; 69: 799-807.
5. Kavanaugh A, Menter A, Mendelsohn A, et al. Effect of ustekinumab on physical function and healthy-related quality of life in patients with psoriatic arthritis: A randomized, placebo controlled, phase Ⅱ trial. Curr Med Res Opin 2010; 26: 2385-92.
6. Kumar N, Narang K, Cressey BD, et al. Long-term safety of ustekinumab for psoriasis. Expert Opin Drug Saf 2013; 12: 757-65.
7. Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate- to-severe plaque-type psoriasis: Long-term results from a phase 2/3 clinical trial. J Dermatol 2012; 39: 242-52.
8. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377-85.
9. Dogra S, Mahajan R. Systemic methotrexate therapy for psoriasis: Past, present and future. Clin Exp Dermatol 2013; 38: 573-88.
10. Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 2007; 356: 580-92.
11. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371: 1665-74.
12. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371: 1675-84.
13. Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo- controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci 2011; 63: 154-63.
14. Zhu X, Zheng M, Song M, et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: Results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol 2013; 12: 166-74.
 Chinese Medical Sciences Journal2014年3期
Chinese Medical Sciences Journal2014年3期
- Chinese Medical Sciences Journal的其它文章
- Clinicopathological Analysis of 155 Patients with Persistent Isolated Hematuria
- Probiotics' Preventive Effect on Pediatric Food Allergy: A Meta-analysis of Randomized Controlled Trials
- Hyperperfusion of Multiple Sclerosis Plaques Characterized by 3D FSE Arterial Spin Labelling
- rmhTNF-α Combined with Cisplatin Inhibits Proliferation of A549 Cell Line In Vitro
- Phosphatase and Tension Homolog Overexpression in Insulin Resistant Diabetic Adipose Tissue△
- Associations Between Epidermal Growth Factor Receptor Gene Mutation and Serum Tumor Markers in Advanced Lung Adenocarcinomas: A Retrospective Study△
