Physico-chemical analysis and antimicrobial potential of Apis dorsata, Apis mellifera and Ziziphus jujube honey samples from Pakistan
Hira Fahim, Javid Iqbal Dasti*, Ihsan Ali, Safia Ahmed, Muhammad Nadeem
1Department of Microbiology, Qauid-i-Azam University Islamabad, Pakistan
2PCSIR Research Laboratories, Thokar Niaz Baig, Lahore, Pakistan
Physico-chemical analysis and antimicrobial potential of Apis dorsata, Apis mellifera and Ziziphus jujube honey samples from Pakistan
Hira Fahim1, Javid Iqbal Dasti1*, Ihsan Ali1, Safia Ahmed1, Muhammad Nadeem2
1Department of Microbiology, Qauid-i-Azam University Islamabad, Pakistan
2PCSIR Research Laboratories, Thokar Niaz Baig, Lahore, Pakistan
PEER REVIEW
Peer reviewer
Viroj Wiwanitkit, Visiting Professor, Hainan Medical University, China; Visiting Professor, Faculty of Medicine, University of Nis, Serbia; Adjunct Professor, Joseph Ayobabalola University, Nigeria. Tel: 6624132436, E-mail: wviroj@yahoo.com
Co-reviewer: Luís Rodrigues da Silva, Porto, Portugal.
Comments
It is an ethnopharmacological research on a locally available honey samples from Pakistan. New data on the properties of studied samples can be seen in the present report. Implication on microbiology is interesting and can be further useful. Further referencing on this work is possible.
Details on Page 640
Objective:To evaluate physico-chemical properties and antimicrobial potential of indigenous honey samples against different reference strains including Escherichia coli ATCC 8739, Enterobacter aerogenes ATCC 13048, Pseudomonas aeroginosa ATCC 9027, Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 25923, Salmonella typhi ATCC 14028, Klebsiella pneumonia ATCC 13883, Aspergillus niger ATCC 16404, Rhizopus oligosporus PCSIR1, Candida albicans ATCC 14053 and Candida utilis ATCC 9950.
Ziziphus jujube honey, Antimicrobial properties, Non-peroxidase activity, Antioxidative properties
1. Introduction
Honey is a heterogeneous mixture of proteins, flower nectar sugars and glandular secretions produced by honey bees[1]. Honey contains significant antioxidant contents including glucoseoxidase, catalase, ascorbic acid, flavonoids, phenolic acids, carotenoid derivatives, organic acids, Maillard reaction products, amino acids and proteins[2-4]. Generally, the darker the honey is, the higher its phenolic content and its antioxidant poweris contained[5,6]. According to a study honey increases antioxidant agents, vitamin C concentration by 47%, β-carotene by 3%, uric acid by 12% and glutathione reductase by 7% in human body[7]. Antioxidant activity depends on the botanical origin of honey and shows variations in different honeys acquired from different sources [4,6,8].
Furthermore, previous studies showed that honey had remarkable antimicrobial activity against fungi, bacteria, viruses and protozoa[9]. This activity of honey has been reported against dermatophytes, some yeast,Aspergillusspp. andPenicilliumspp.[9]. Moreover, it has been shown that honey has inhibitory effects on rubella virus, herpes virus and three species of theLeishmaniaparasite[9,10]. Antimicrobial activity of honey is linked to hydrogen peroxide which is produced by glucose oxidase especially when honey is diluted. In addition, hydrogen peroxide has antibacterial activity and at the same time it is not tissue damaging[11].
In the diluted form of honey, produced hydrogen peroxide is an important stimulant of the growth of tissues and has the potential for wound healing[12]. The hydrogen peroxide activity of most of the honeys can be destroyed by heat or by the presence of catalase. However, some honeys retain their antimicrobial activity even in the presence of catalase which are known as “non-peroxidase honeys”[9]. This activity is important especially in the context of topical antimicrobial and wound dressings fluids[13].
Recently some progress has been made regarding the treatment of infections caused by methicillin-sensitiveStaphylococcus aures. However, due to different methodologies and variety of honey samples used data are inconsistent, which suggests the need to further evaluate potency of different honey samples. To our understanding, little is known about the antimicrobial potential of a particular type of honeys indigenous to Pakistan. This study focused on the analysis of different honey samples. Prior to antimicrobial activity testing, the physico-chemical properties (such as pH, color, ash contents, protein contents, moisture contents, hydroxymethyl furfural contents, total sugar contents, reducing sugar and non-reducing sugar contents), antimicrobial properties (such as additive effect of starch and non-peroxidase activity) and antioxidative properties (such as phenol contents, flavonoid contents and 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity) of various honey samples were analyzed to assess the quality of these honey samples.
2. Materials and methods
2.1. Honey samples
Three different types of honey samples includingApisdorsata(locally called big honey),Apis mellifera(small honey), andZiziphus jujube(Siddar Honey) were collected from a local honey centre in Lahore, Pakistan and the experimental work was conducted in PCSIR laboratories Lahore, Pakistan.
2.2. Physico-chemical analysis of honey
The pH was determined with a digital pH meter by using the method described by the Association of Official Analytical Chemist[14]. The color of the honey samples was determined by spectrophotometric measurement of the absorbance of 50% (w/v) honey solution at 635 nm according to the method of White[15]. The color of honey samples were classified according to the Pfund scale after conversion of the absorbance values.
2.3. Ash, moisture and total protein content measurements
Total ash contents were measured by incinerating honey samples in a muffle furnace at a temperature of 550 °C according to AOAC Official Method[16]. The total protein content was determined spectrophotometrically by using Bovine Serum Albumin (BSA) standard curve according to Lowry’s method (1951). The moisture content was determined by using refractometric method given in the harmonised methods of the European Honey Commission[17]. The refractive index values were further corrected for a standard temperature of 20 °C by adding the correction factor of 0.000 23/°C. The moisture values corresponding to the corrected refractive indices values were calculated using the Chataway Table[18].
2.4. Effect of heat treatment on hydroxymethyl furfural (HMF) production
The HMF contents were estimated spectrophotometrically by using the method of White at different temperatures (60 °C, 70 °C and 80 °C)[19].
2.5. Estimation of total sugar and reducing sugar content
Total sugar content was determined spectrophotometrically according to previously described method of Duboiset al.by using sucrose to plot calibration curve[20]. The estimation of reducing sugar content was done spectrophotometrically according to dinitrosalicylic acid method proposed by Miller[21]. Glucose was used as a standard for preparing the calibration curve. The amount of non-reducing sugars, such as sucrose content, was measured by subtracting the reducing sugar content from total sugar content as previously described[22].
2.6. Estimation of total phenol and flavonoid contents
The total phenol contents were determined spectrophotometrically by using Folin-Ciocalteu reagent according to the method of Singletonet al[23]. Vanillin was used as a standard for preparing the calibration curve. The blue complex was formed by the reduction of the Folin-Ciocalteu reagent by phenolic compounds present in honey. The total flavonoid contents were determined spectrophotometrically according to the method of Zhishenet al[24]. Catechin was used as a standard for preparing the calibration curve.
2.7. Measurement ofDPPHradical scavenging activity and non-peroxidase activity
The scavenging activity of honey samples for the radical 1,1-diphenyl-2-picrylhydrazyl (DPPH) was measured as described by Velazquezet al[25]. The nonperoxidase antimicrobial activity of honey was determined spectrophotometrically by using 0.2% (w/v) catalase solution according to the method of Sherlocket al.[26], using 96-well microtitre plate. The minimum bactericidal concentration (MBC) was determined by sub-culturing a loopful of the culture media on sterilized nutrient agar plate (from each test well that showed no apparent growth). After incubation, the MBC was read as the least concentration showing no growth on the nutrient agar plates. After that, 0.2% (w/v) catalase solution was added in each honey dilution and it was incubated in dark at room temperature for 24 h. Honey dilution containing catalase was mixed with microbial culture. Control wells include negative control (wells containing nutrient broth), positive control (wells containing inoculums and nutrient broth) and the corresponding negative control (wells containing honey dilution and nutrient broth). After incubation (2 d at 37 °C for bacteria and 5 d at 25 °C for yeast and fungal strains), growth was observed by visual inspection and by measuring the optical density at 620 nm with the help of plate reader (Asys, VUM-340). The MBC was determined by sub-culturing a loopful of the culture media on sterilized nutrient agar plate (from each test well that showed no apparent growth). After incubation, the MBC was read as the least concentration showing no growth on the nutrient agar plates.
2.8. Antimicrobial activity of honey
For well diffusion assay, different honey concentrations against selected microbes were used according to the method of Al-Somalet al[11]. The honey samples were tested at 20 different concentrations ranging from 5% to 100% (v/ v). Sterilized agar media was inoculated with 24 h old microbial culture and was poured in sterilized plates. After solidification, wells were made by using sterilized borer. Honey dilutions were poured in each well aseptically. After incubation, zones of inhibition were noted in millimeter with the help of a scale. The synergistic effect of starch on the antimicrobial activity of honey was performed spectrophotometrically by using 10% (w/v) starch solution.
3. Results
3.1. Estimation of pH, colour ash, protein moisture and sugar content
The pH of tested honey samples remained 3.14 to 4.19 throughout this study. As is shown in Table 1 that the Siddar honey had the highest pHi.e.4.190±0.707 in comparison to other honey samples. The color of the tested honey samples falls between amber to dark amber, particularly Siddar honey showed the dark amber color (Table 1). The total ash contents in tested honey samples ranged between 0.411%-0.590%. The highest ash contents were found in the Siddar honeyi.e.(0.590 0±0.033 6)%. The total protein contents in three types of honey samples used in this study ranged from 571.272 to 777.598 mg/kg (Table 1). The small honey had the highest protein contentsi.e.(777.598±9.880) mg/kg. However lowest protein contents were found in the big honeyi.e.(571.272±6.020) mg/kg. The moisture contents in tested honey samples ranged from 13.800%-16.600% (Table 1). The highest moisture contents were found in the Siddar honeyi.e.(16.600%±0.283)%. It was observed that the HMF contents increases with the increase in temperature (Table 2). The range of HMF contents in unheated honey samples was from 27.69-36.08 mg/kg. The highest HMF contents were found in the small honeyi.e.(36.08±1.06) mg/kg. It was observed that when the temperature was increased, the HMF contents were also increased (Table 2).
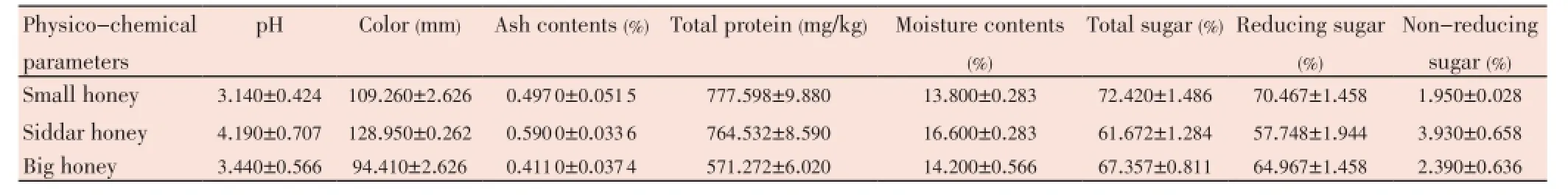
Table 1 Physico-chemical characteristics of three different types of honey samples (mean±SD).
The total sugar contents range from 61.672%-72.420% (Table 1). In this study, the highest sugar contents were found in the small honeyi.e.(72.420±1.486)%. The reducing sugar contents ranges from 57.748%-70.467% (Table 1). Regarding reducing sugars (fructose and glucose), EU Directive[27], imposes values ≥60% and according to the obtained results, only one sample (Siddar honey) showed a value that was lower (57.748%) in comparison to the standard value set for reducing sugars by EU Directive[27]. The non-reducing sugar contents were found to be in the range of 1.950%-3.930% (Table 1). Finally, the highest non-reducing sugar contents were found in the Siddar honeyi.e.(3.930±0.658)%.
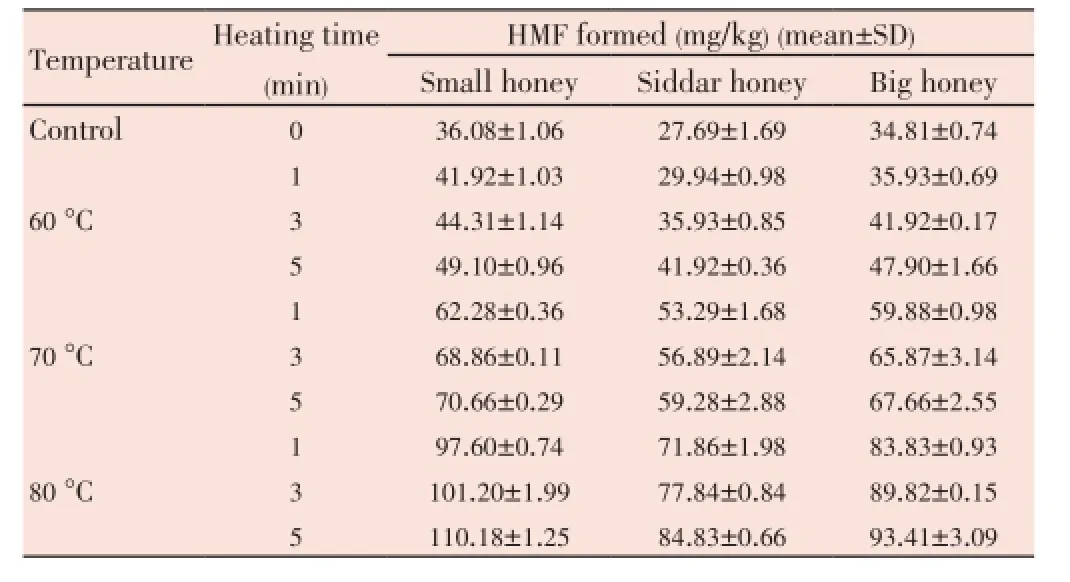
Table 2 Effect of temperature on HMF production in different honey samples.
3.2. Phenolic and flavonoid content and DPPH radical scavenging activity
The total phenolic contents in all honey samples were determined and it was observed that the total phenolic contents in our tested honey samples range from 460.260 to 540.720 mg/kg (Table 3). The highest phenolic contents were present in the Siddar honeyi.e.(540.720±3.035) mg/kg. Similarly, the total flavonoid contents were observed in the range of 46.519-64.396 mgcatechin/kg (Table 3). The highest flavonoid content was found in the Siddar honeyi.e.(64.396± 3.534) mg/kg. The DPPH radical scavenging activity of honey at different concentrations was studied and it was observed that the Siddar honey has slightly higher radical scavenging activity as compared to other two honey samples (Figure 1).
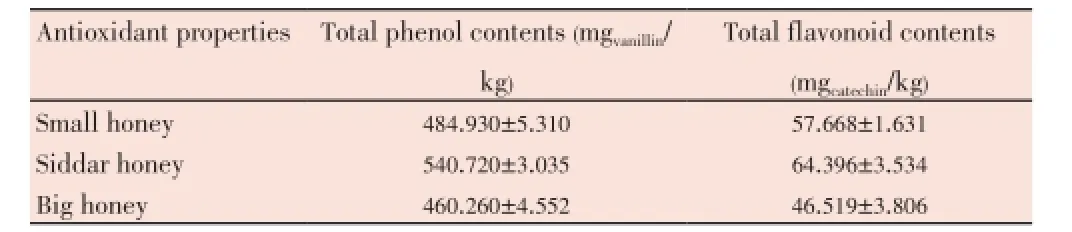
Table 3 Total phenol and flavonoid contents in some Pakistani honey samples.
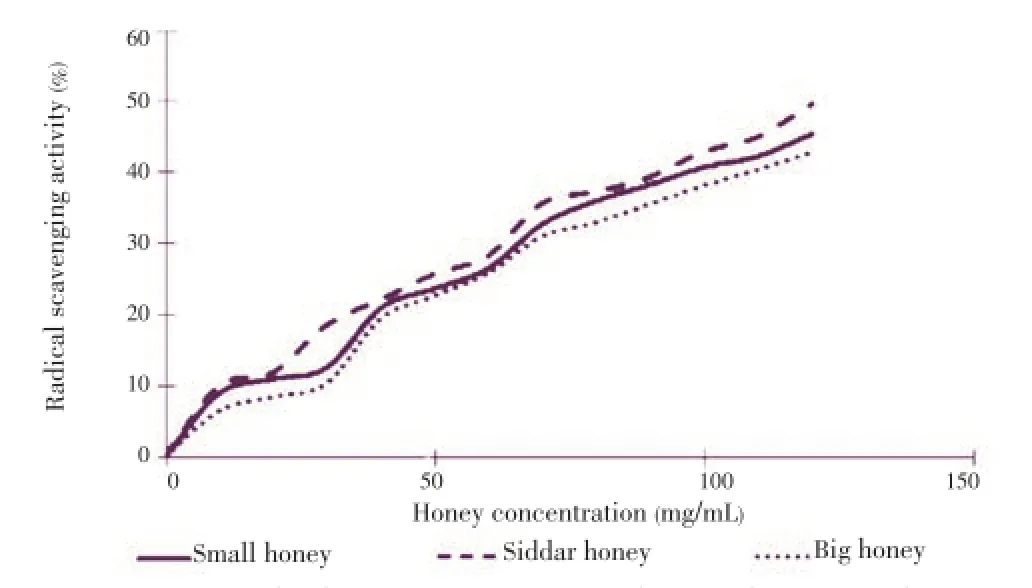
Figure 1. DPPH radical scavenging activity of different honey samples.
3.3. Antimicrobial activity of honey
The well diffusion assay was performed to measure the zones of inhibition produced by using different concentrations of honey samples against various pathogenic microorganisms and results are shown in Figure 2. It was observed that all bacteria showed clear inhibition zones in response to all honey samples whereas fungi and yeast showed inhibition at higher concentrations of honey. It is shown in Figure 2 that forEscherichia coli(E. coli),Bacillus subtilis(B. subtilis),Salmonella typhi(S. typhi),Pseudomonas aeroginosa(P. aeroginosa) andAspergillus niger(A. niger),the small honey showed the higher activity than other honey samples. ForEnterobacter aerogenes(E. aerogenes),Staphylococcus aureus(S. aureus),Candida albicans(C. albicansand),Candida utilis(C. utilis), both small and Siddar honey samples showed high activity than big honey. ForKlebsiella pneumonia(K. pneumonia), big honey showed comparatively higher activity. ForRhizopus oligosporus(R.oligosporus), Siddar honey showed higher activity. Overall, it was noticed that Gram-negative microbes were more susceptible to honey as compared to Gram-positive microbes.
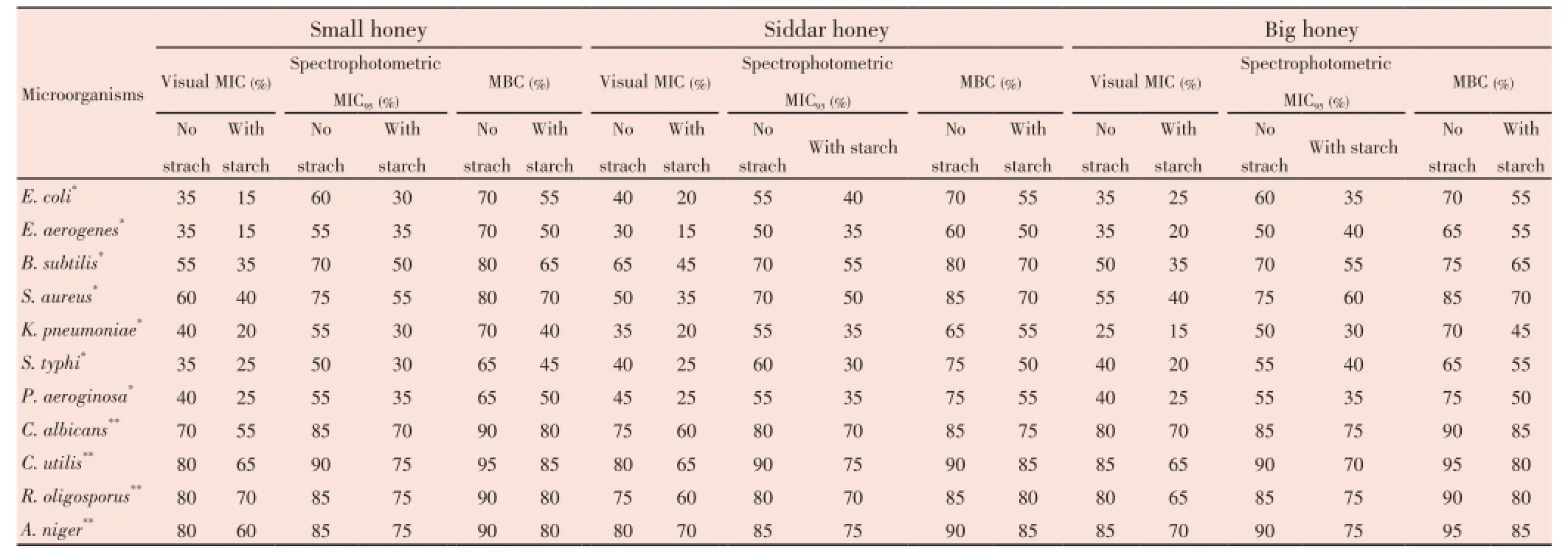
Table 4 Visual MIC, spectrophotometric MIC95and MCB values of different Pakistani honey samples with and without adding starch in them.
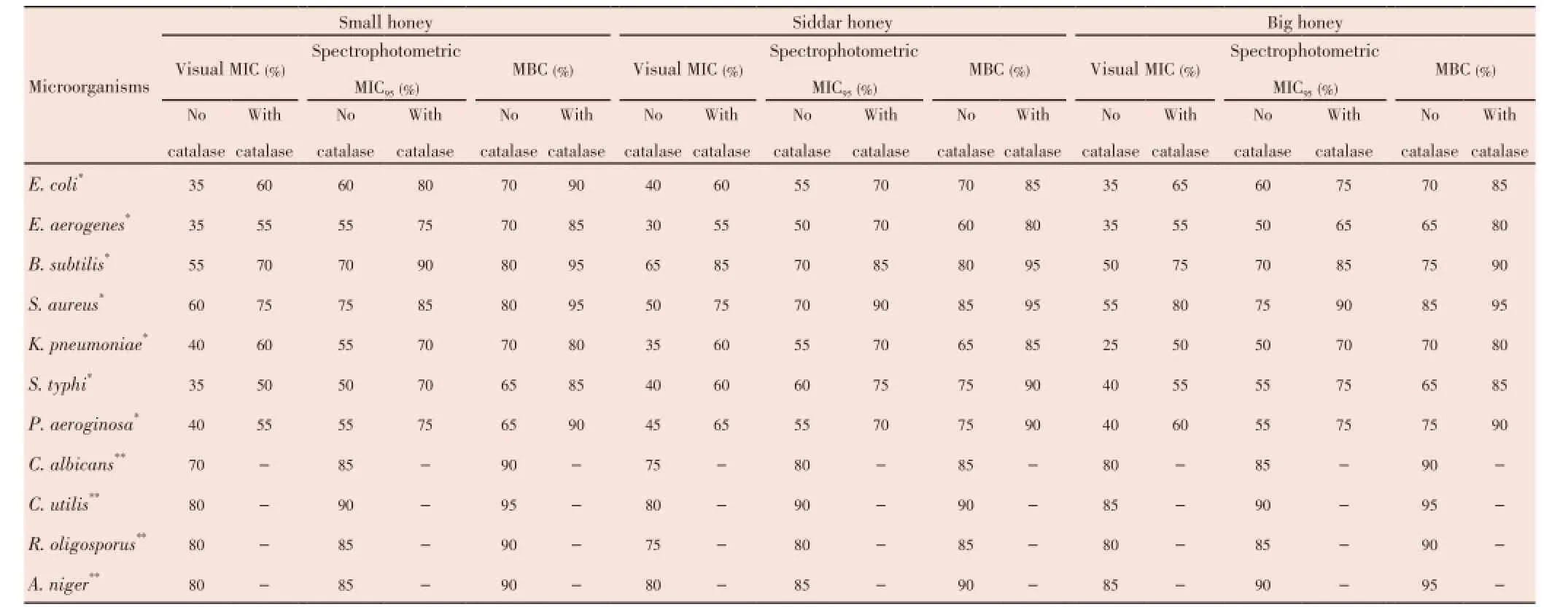
Table 5 Visual MIC, spectrophotometric MIC95and MCB values of different Pakistani honey samples with and without adding catalase in them.

Figure 2. The inhibition zone diameters of tested microorganisms.Straight line: Small honey; Dotted line: Siddar honey; Dashed line: Big honey.
Upon addition of starch the antimicrobial potential was increased (Table 4). However, when starch solution was added in honey, the MIC values were decreased. Upon mixing honey samples with starch, small honey showed more effectiveness againstE. coliandB. subtilis. Both small and Siddar honey seems to be more effective againstE. aerogenes,S. typhiandC. albicans. Similarly, Siddar honey showed more effectiveness againstS. aureusandR. oligosporus. However, big honey showed more effectiveness againstC. utilis. Both small and big honey samples showed more effectiveness againstK. pneumonia. All honey samples showed same activity againstP. aeroginosaandA. niger.
3.4. Non-hydrogen peroxidase activity of honey sample
The non-hydrogen peroxidase activity of all honey samples were studied spectrophotometrically (Table 5). Upon removal of hydrogen peroxide from the honey samples, the MIC values were increased. In the absence of hydrogen peroxide, Siddar honey showed more effectiveness againstE. coliandP. aeroginosa. Big honey seems to be relatively more effective againstE. aerogenesand the small honey againstS. aureusandS. typhi. Both Siddar and big honey samples showed more effectiveness againstB. subtilusand all honey samples showed same activity againstK. pneumonia.
4. Discussion
Physico-chemical analysis of all honey samples confirmed acidic pH ranging from 3.14 to 4.19. Low pH of honey is attributed to the presence of organic acids such as gluconic, pyruvic, malic and citric acids[28], and the variation in pH of different honey samples is described to be due to floristic composition and floral diversity of the regions. Observed values in this study are lower than those previously reported for other honey samples from India, which had pH values between 3.7 and 4.4[29]. Published data suggests that pH of honey may range between 3.2 and 4.5 and our finding is approximately within this range[30]. Overall however, the Siddar honey has the highest pHi.e.(4.190±0.707) that might be due to the presence of alkaline contents in Siddar extracts. Similar findings were made in Saudi Arabia for sidr honey by Abu-Tarboush[31].
In this study the colors of all samples fall between amber to dark amber. The Siddar honey showed the dark amber color. Batrusaityteet al.reported that the color of honey is usually related to the mineral, pollen and phenolic contents of honey[32]. Jasicka-Misiaket al.also reported that the higher levels of polyphenols in honey resulted in the darker color of honey[33]. The total phenolic contents of the honey samples used in this study are higher than those of two reported Malaysian honey samples as well as that of Gelam and Coconut honeys, which are lighter in color[3]. Total ash contents in tested honey samples ranged between 0.411% and 0.590%. These findings meet the standards of European Union Commission[34], and Codex Alimentarius Commission[35], and are comparable with other studies like Salimet al.[36], in which ash contents ranged up to 0.09-0.54. Relatively higher ash contents were found in the Siddar honeyi.e., 0.590 0%±0.033 6%. Similar findings were made by Abu-Tarboushet al.[31] who found the highest ash contents in sidr honey. Furthermore, total protein contents in three honey samples used in this study ranged between 571.272 and 777.598 mg/kg. Comparatively, small honey showed highest protein contentsi.e.(777.598±9.880) mg/kg and the lowest protein contents were found in the big honeyi.e.(571.272±6.020) mg/kg. These findings are much more different than those observed by Islamet al.[37], who found the range of protein contents in Bangladeshi honey samples remained between 900 mg/kg and 8 600 mg/kg. The moisture contents of tested honey samples ranged between 13.8% and 16.6%, which is in the range defined by Egyptian Organization Standards[38], European Union Commission[34], and Codex Alimentarius Commission[35]. Almost similar finding was reported by Khalilet al.[22], who found the moisture contents in their tested honey samples ranging between 11.59% and 14.13%. In this study, the highest moisture contents were found in the Siddar honeyi.e.(16.600±0.283)% which is lower than the maximum suggested moisture contents by international standards. The percent moisture contents of analyzed honey samples tends to be less than other investigated honeys, such as 17.19%-19.19% for samples from Bangladesh[37], 17.2%-21.6% for samples from India[29] and 17.0%-19.4% for samples from Turkey[8]. Water content is very important for the shelf-life of honey during storage and can lead to undesirable honey fermentation due to osmotolerant yeasts, which form ethyl alcohol and carbon dioxide[17].
In this study a direct increase in the HMF contents was observed by increasing temperature. The range of HMF contents in unheated honey samples was from 27.69-36.08 mg/kg. This result was within the recommended range set by the Codex Alimentarius at 80 mg/kg[39]. The valuesare also within the allowed maximum limit of 40 mg/kg, as recommended by the Turkish Alimentarus Codex for honey samples from tropical countries[40]. However in some studies tested honey samples showed very low HMF contentsi.e.0.2-22.8 mg/kg[41]. On contrast, Khalilet al.found that Malaysian Tualang honey samples have very high HMF concentrationsi.e.118.47-1139.95 mg/kg[42]. Overall, the low HMF concentrations of these tested honey samples confirmed good quality of the samples. It was observed that when the temperature was increased, the HMF contents were also increased. Similar observations were made by Tosiet al.who recorded that HMF increased from 10.1 ppm to 32.8 ppm by heating of honey for 1 min at 100 °C and 140 °C respectively[43]. In addition, This increase in HMF at high temperatures is associated with the decomposition of labile fructose particularly in the presence of acid[44]. For the analyzed honey samples total sugar contents range from 61.672% to 72.42%. None of the samples exceeded the highest limit set for total sugar content by the European community directive[27]. Regarding reducing sugars (fructose and glucose), EU Directive imposes values ≥60% and according to the obtained results[27], only one sample (Siddar honey) showed a value lower (57.748%) as compared to the standard value set for reducing sugars by EU Directive[27]. Borsatoet al.also found lower reducing sugar content in their one honey samplei.e.58.75%[45]. Overall our results are comparable to the results obtained by Gomeset al.who found the range of reducing sugar contents in organic honey from the northeast of Portugal that ranged between 65.60% and 68.90%[46]. These finding are also comparable with the results obtained by Borsatoet al.who found the range of reducing sugar contents of honeys of Campos Gerais region of Paraná, Brazil that ranged between 58.75% and 82.37%[45]. The non-reducing sugar contents were found to be in the range of 1.95%-3.93% the highest non-reducing sugar contents were found in the Siddar honeyi.e.(3.930±0.658)%. This might be due to the early harvest of the honey. Gomeset al.reported that the higher sucrose contents could be the result of an early harvest of honeys[46],i.e.as sucrose has yet to be converted to fructose and glucose.
Like non-reducing sugar content the highest phenolic contents were present in the Siddar honeyi.e.(540.720± 3.035) mg/kg which indicates its high antioxidant potential. The highest flavonoid content was reported in the Siddar honeyi.e.(64.396±3.534) mg/kg. The flavonoid contents of our tested honey samples were higher than those of Turkish[47], and Malaysia honey samples[48], which ranged from 4.80-22.80 mgcatechin/kg and 11.52-25.31 mgcatechin/kg respectively, which indicates Siddar honey samples have higher antioxidant potential as compared to Turkish and Malaysian honey samples. The DPPH radical scavenging activity of honey at different concentrations was checked and it was observed that the Siddar honey has slightly higher radical scavenging activity as compared to other two honey samples. Possibly, observed high radical scavenging activity of the Siddar honey is due to the high phenolic and flavonoid contents in it, thus indicating high antioxidant potential. Furthermore, it was observed that the highest radical scavenging activity is linked with the highest honey concentration tested of all honey samples. Recently, similar findings were reported by Khalilet al.who found the highest scavenging activity at highest honey dilution[22]. The percentage of inhibition exhibited by tested honey samples were similar to that of Algerian honey samples[22], some Malaysian honey samples and Indian honey samples[29,48].
The non-hydrogen peroxidase activity of all honey samples was checked that showed removal of hydrogen peroxide increases the MIC values. In the absence of hydrogen peroxide, Siddar honey showed more effectiveness againstE. coliandP. aeroginosa. Big honey showed more effectiveness againstE. aerogenes. Small honey showed more effectiveness againstS. aureusandS. typhi. Overall, both Siddar and big honey samples showed more effectiveness againstB. subtilus. All honey samples showed same activity againstK. pneumonia. However, all honey samples showed no non-peroxidase activity againstC. albicans,C. utilis,R. oligosporusandA. niger.
All bacteria showed clear inhibition zones in response to all honey samples whereas fungi and yeast showed inhibition at higher concentrations of honey. ForE. coli,B. subtilis,S. typhi,P. aeroginosaandA. niger, the small honey showed higher activity than other honey samples. ForE. aerogenes,S. aureus,C. albicansandC. utilis, both small and Siddar honey samples showed higher activity than big honey. ForK. pneumonia, big honey showed comparatively higher activity. ForR.oligosporus, Siddar honey showed higher activity. Allenet al.reported the antibacterial properties of honey against two laboratory isolatese.g.P. aeroginosaandE. coli[1]. El-Toum and Yagoub found through well diffusion method that sidr honey showed antimicrobial activity againstS. aureus[49],K. aerogenes,P. aeruginosaandC. albicans, and the zone of inhibition ranged between 9 mm and 50 mm, and sunflower honey showed markedly sensitivity towardsE. coli,S. aureusandK. aerogenes, and the inhibitions zone were between 15 mm and 50 mm, and sunut honey showed antimicrobial activity towardS. aureus,E. coli,K. aerogenesandC. albicans, and the inhibition zone range between 10 mm and 42 mm. It was also noticed that Gram-negative bacteria were more susceptible to honey as compared to Gram-positive bacteria.
This study confirms antimicrobial properties of these honey samples againstE. coli,E. aerogenes,P. aeruginosa,B. subtilis,S. aureus,S. typhi,K. pneumonia,A. niger,Rhizopus oligosporus,C. albicansandC. utilisATCC 9950.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgement
We thank Higher Education Commission Pakistan for partly supporting this study (via IPFP Grant No. 3782) and PCSIR Laboratories Lahore for technical support.
Comments
Background
It is an ethnopharmacological research on a locally available honey sample made from local species that can be seen in some tropical area of Pakistan.
Research frontiers
A standard ethnopharmacology study on locally available honey samples. It can be a good data for further pharamaceutical and pharmacological research.
Related reports
Not much on the studied samples. However, there are some previous publications from Pakistan and Middle East on the locally available honey such as ones from Iraq.
Innovations and breakthroughs
New data on the properties of studied samples can be seen in the present report. Implication on microbiology is interesting and can be further useful.
Applications
Data can be further referred and used in further pharmaceutical and pharmacological research. Also, the test on microbiology aspect is helpful in tropical medicine work.
Peer review
It is an ethnopharmacological research on a locally available honey samples from Pakistan. New data on the properties of studied samples can be seen in the present report. Implication on microbiology is interesting and can be further useful. Further referencing on this work is possible.
[1] Allen KI, Radwan S, Reid GM. Antimicrobial activity of honey on some microbial isolates. J Med Pharm Sci 2000; 2: 75-79.
[2] Beretta G, Granata P, Ferrero M, Orioli M, Facino RM. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal Chim Acta 2005; 533(2): 185-191.
[3] Aljadi AM, Kamaruddin MY. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem 2004; 85(4): 513-518.
[4] Al-Mamary M, Al-Meeri A, Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutr Res 2002; 22: 1041-1047.
[5] Al ML, Daniel D, Moise A, Bobis O, Laslo L, Bogdanov S. Physicochemical and bioactive properties of different floral origin honeys from Romania. Food Chem 2009; 112(4): 863-867.
[6] Vela L, de Lorenzo C, Pérez RA. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J Sci Food Agric 2007; 87(6): 1069-1075.
[7] Al-Waili NS. Effects of daily consumption of honey solution on hematological indices and blood levels of minerals and enzymes in normal individuals. J Med Food 2003; 6(2): 135-140.
[8] Kü?ük M, Kolayl? S, Karao?lu S, Ulusoy E, Baltac? C, Candan F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem 2007; 100: 526-534.
[9] Molan PC. The antibacterial activity of honey. 1. The nature of the antibacterial activity. Bee World 1992; 73(1): 5-28.
[10] Al-Waili NS. Topical honey applications vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med Sci Monit 2004; 10(8): MT94-MT98.
[11] al-Somal N, Coley KE, Molan PC, Hancock BM. Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J R Soc Med 1994; 87(1): 9-12.
[12] White JW Jr, Subers MH, Schepartz AI. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim Biophys Acta 1963; 73: 57-70.
[13] Molan PC, Allen KL. The effect of gamma-irradiation on the antibacterial activity of honey. J Pharm Pharmacol 1996; 48(11): 1206-1209.
[14] AOAC. Sugars and sugar products. In: Horwitz W, editor. Official methods of analysis of AOAC international. Maryland, USA: AOAC International; 1990, p. 22-33.
[15] White JW. Instrumental color classification of honey: collaborative study. J Assoc Off Anal Chem 1984; 67: 1129-1131.
[16] AOAC. Determination of ash in honey. In: Gaithersburg MD, editor. Official methods of analysis of AOAC international. Maryland, USA: AOAC International; 1996.
[17] Bogdanov S, Martin P, Lüllmann C. Harmonised methods of theEuropean honey commission. Apidologie 1997; 28: 1-59.
[18] Chataway HD. Honey tables showing the relationship between various hydrometer scales and refractive index to moisture content and weight per gallon of honey. Can Bee J 1935; 43: 215.
[19] White JW Jr. Spectrophotometric method for hydroxymethylfurfural in honey. J Assoc Off Anal Chem 1979; 62(3): 509-514.
[20] Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem 1956; 28: 350-356.
[21] Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 1959; 31: 426-428.
[22] Khalil MI, Moniruzzaman M, Boukraa L, Benhanifia M, Islam MA, Islam MN, et al. Physicochemical and antioxidant properties of Algerian honey. Molecules 2012; 17: 11199-11215.
[23] Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 1999; 299: 152-178.
[24] Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64: 555-559.
[25] Velázquez E, Tournier HA, Mordujovich de Buschiazzo P, Saavedra G, Schinella GR. Antioxidant activity of Paraguayan plant extracts. Fitoterapia 2003; 74: 91-97.
[26] Sherlock O, Dolan A, Athman R, Power A, Gethin G, Cowman S, et al. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Compl Altern Med 2010; 10: 47.
[27] European Parliament, Council of the European Union. Directive 2002/22/EC of the European parliament and of the council of 7 March 2002. European Union: European Parliament; 2002. [Online] Available from: http://eur-lex.europa.eu/legal-content/EN/ ALL/?uri=CELEX:32002L0022 [Accessed on 26th October, 2013]
[28] Bogdanov S, Ruoff K, Oddo LP. Physico-chemical methods for the characterization of unifloral honeys: a review. Apidologie 2004; 35(Suppl 1): S4-S17.
[29] Saxena S, Gautam S, Sharma A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem 2010; 118: 391-397.
[30] Bogdanov MB. Brightness distributions for models of circumstellar dust shells of cool carbon stars. Astron Astrophys Trans 1995; 8: 23-29.
[31] Abu-Tarboush HM, Al-Khatani HA, El-Sarrage MS. Floral-type identification and quality evaluation of some honey types. Food Chem 1993; 46: 13-17.
[32] Baltru?aityt? V, Venskutonis PR, ?eksteryt? V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem 2007; 101(2): 502-514.
[33] Jasicka-Misiak I, Poliwoda A, Dereń M, Kafarski P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish uni floral honeys. Food Chem 2012; 131: 1149-1156.
[34] European Union Commission. Proposal for a directive of the European council relating to honey. EUD Document 96/0114. Bruxelles, Belgium; 1996.
[35] Codex Alimentarious Commission. Codex alimentarious draft revised for honey CAD CX P 5/102, CI, 1998/12-S 1998. Rome, Italy: FAO; 1998.
[36] Ouchemoukh S, Louaileche H, Schweitzer P. Physicochemical characteristics and pollen spectrum of some Algerian honeys. Food Control 2007; 18: 52-58.
[37] Islam A, Khalil I, Islam N, Moniruzzaman M, Mottalib A, Sulaiman SA, et al. Physicochemical and antioxidant properties of Bangladeshi honeys stored for more than one year. BMC Complement Altern Med 2012; 12: 177.
[38] Egyptian Organizition Standards. Egyptian honey standards: inspecting and testing. EOS Part I: Bee Honey. EOS No 355 P1. Cairo, Egypt: Egyptian Organizition Standards; 1990.
[39] Codex Alimentarius Commission. Draft revised standard for honey at step 8 of the codex procedure; Rome, Italy: FAO; 2000. [Online] Available from: http://www.fao.org/docrep/meeting/005/x4616e/ x4616e0b.htm [Accessed on 2nd May, 2013]
[40] [Honey rescript’s Turkish alimentarus codex]. Turkey: The Official Gazette of the Republic of Turkey; 2003. Turkish.
[41] Feás X, Pires J, Estevinho ML, Iglesias A, de Araujo JPP. Palynological and physicochemical data characterisation of honeys produced in the Entre-Douro e Minho region of Portugal. Int J Food Sci Technol 2010; 45: 1255-1262.
[42] Khalil MI, Sulaiman SA, Gan SH. High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. Food Chem Toxicol 2010; 48: 2388-2392.
[43] Tosi E, Ciappini M, Ré E, Lucero H. Honey thermal treatment effects on hydroxymethylfurfural content. Food Chem 2001; 77: 71-74.
[44] Rodgers PEW. Honey quality control. In: Crane E, editor. Honey: a comprehensive survey. London, UK: Heinemann; 1979, p. 314.
[45] Borsato DM, Vargas T, Koop L, Farago PV, de Almeida MM. Physicochemical quality control of bee honeys from campos gerais region of Paraná-Brazil. Boletim do Centro de Pesquisa e Processamento de Alimentos 2010; 28(2): 205-212.
[46] Gomes T, Feás X, Iglesias A, Estevinho LM. Study of organic honey from the northeast of Portugal. Molecules 2011; 16: 5374-5386.
[47] ?zk?k A, D’arcy B, Sorkun K. Total phenolic acid and total flavonoid content of Turkish pine honeydew honey. J ApiProduct ApiMedical Sci 2010; 2: 65-71.
[48] Khalil MI, Mahaneem M, Jamalullail SMS, Alam N, Sulaiman SA. Evaluation of radical scavenging activity and colour intensity of nine Malaysian honeys of different origin. J ApiProdut ApiMedcal Sci 2011; 3: 4-11.
[49] El-Toum SK, Yagoub SO. Compression study of anti-microbial activity of honey-bees. Res J Microbiol 2007; 2: 776-781.
10.12980/APJTB.4.2014APJTB-2014-0095
*Corresponding author: Javid Iqbal Dasti, Department of Microbiology, Qauid-i-Azam University Islamabad, Pakistan.
Tel: 092-51-90643040
E-mail: iqbal78@hushmail.com
Foundation Project: Supported partly by Higher Education Commission Pakistan, (via IPFP Grant No.3782).
Article history:
Received 10 Jun 2014
Received in revised form 20 Jun, 2nd revised form 25 Jun, 3rd revised form 3 Jul 2014
Accepted 13 Aug 2014
Available online 28 Aug 2014
Methods:By using standard methods samples were evaluated for their antimicrobial properties including additive effect of starch and non-peroxidase activity, antioxidative properties (phenol contents, flavonoid contents, 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity). Prior to this evaluation, complete physico-chemical properties including pH, color, ash contents, protein contents, moisture contents, hydroxymethyl furfural contents, total sugar contents, reducing sugar and non-reducing sugar contents were analyzed.
Results:Relatively higher ash contents were found in the Siddar honey i.e. (0.590 0±0.033 6)% and small honey showed relatively higher protein contents i.e. (777.598±9.880) mg/kg. The moisture contents of tested honey samples ranged between 13.8%-16.6%, total sugar contents from 61.672%-72.420% and non-reducing sugar contents from 1.95%-3.93%. Presences of phenolic contents indicate higher antioxidant potential of these honey samples. All bacteria showed clear inhibition zones in response to tested honey samples whereas fungi and yeast showed inhibition at higher concentrations of these honey samples. For Escherichia coli, Bacillus subtilis, Salmonella typhi, Pseudomonas aeroginosa and Aspergillus niger, overall the small honey showed the higher activity than other honey samples.
Conclusion:Physico-chemical analysis of honey samples confirmed good quality of honey according to the standards set by European Union Commission and Codex Alimentarius Commission. Evaluation of these honey samples confirms antimicrobial potential of particular types of honeys indigenous to Pakistan.
 Asian Pacific Journal of Tropical Biomedicine2014年8期
Asian Pacific Journal of Tropical Biomedicine2014年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- An overview of travel-associated central nervous system infectious diseases: risk assessment, general considerations and future directions
- Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates along the Thai-Myanmar borders
- Impact of antibacterial drugs on human serum paraoxonase-1 (hPON1) activity: an in vitro study
- High levels of Zinc-α-2-Glycoprotein among Omani AIDS patients on combined antiretroviral therapy
- Toxicity effects of water extracts of Holothuria atra Jaeger in mice
- Prevention of renal dysfunction by nutraceuticals prepared from oil rich plant foods
