Mechanism of T cell regulation by microRNAs
Juan Liu, Chang-Ping Wu, Bin-Feng Lu, Jing-Ting Jiang
1Department of Tumor Biological Treatment, The Third Affiliated Hospital of Soochow University, Changzhou 213003, China;2Department of Immunology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA
Introduction
Cancer progression is associated with many gene mutations and abnormal gene expressions.As a result, tumor-specific and tumor-associated antigens, which can stimulate T cell immune responses against malignant cells, are produced1.Immune responses to tumors consist of three stages, namely, elimination,equilibrium, and escape2.In the elimination stage, cancer cells are recognized and eradicated by the immune system.If the immune system fails to eradicate tumor cells, the equilibrium stage is reached.In the equilibrium stage, tumor cells and immune cells coexist.In the escape stage, tumors grow by developing immune suppressive mechanisms to escape immune attack mediated primarily by type 1 immune cells.Hence, new approaches should be developed to fight cancer by investigated the exact mechanism by which microRNAs regulate the functions of T cells3,4.
Non-coding RNAs are indirectly involved in translation,particularly in regulating protein synthesis.These RNAs have several types, including small RNAs and long non-coding RNAs (lncRNAs).Similar to mRNA, lncRNAs are more than 200 nucleotides (nt) in length with a 5’ methyl cap and a polyA tail.However, small RNAs are less than 200 nt; these small RNAs include tRNA, rRNA, snRNA, siRNA, and microRNA(miRNA).MiRNAs are an important class of endogenously expressed small non-coding RNAs.A functional mRNA-targeting mature miRNA is single stranded and typically 19nt to 22nt in length after this type of miRNA is synthesized from double-stranded RNA5-7; mature miRNA also exhibits critical regulatory functions by modulating the rate of protein synthesis in eukaryotes8.
MiRNAs are initially transcribed by RNA polymerase II using a specific genomic DNA as template in cell nuclei.In this process,primary miRNA (pri-miRNA) sequences are produced, in which a hairpin sequence containing the mature miRNA is found.The hairpin of the pri-miRNA was further transformed into precursor miRNAs by the enzyme Drosha and then exported into the cytoplasm via Exportin V.Pre-miRNAs are cleaved into two individual strands of RNA by the enzyme Dicer.One strand of the miRNA is usually degraded and the other strand is associated with RNA-induced silencing complexes (RISC)9.
MiRNA molecules regulate gene expression mainly by binding to complementary sequences in the 3′-untranslated region (UTR) of target mRNAs and then integrating into RISC to suppress translation or to degrade miRNA-bound mRNA transcripts10,11.The functional significance of miRNAs is initially demonstrated in the developmental process of Caenorhabditis elegans12.Further studies have provided a strong link between miRNA and immune system function.
T cells are derived from lymphoid stem cells in the bone marrow and mature in the thymus.Based on the expression of surface molecules, such as CD3, CD4, and CD8, the development of T cells in thymus is divided into three stages: initial stage, in which T cells are formed as double negative cells (CD4–CD8–);intermediate stage, in which T cells are developed as double positive cells (CD4+CD8+); and final stage, in which T cells are formed as single positive cells (CD4–CD8+or CD4+CD8–)13.Based on maturation status, mature T cells are divided into naive cells, effector cells, memory T cells, and exhausted/anergic cells; each subset expresses specific surface molecules, including CCR7, CD45RA, CD70, and CD2714,15.Based on cytokine pro files, T helper cells (Th) can be divided into Th1, Th2, Th17,and Th9 cells, which enhance the activity of other immune cells by producing various cytokines16,17.T cells function in several processes, mainly in recognizing allergens and secreting cytokines that eradicate foreign molecules and cancer cells.For example,CD8+T cell secretes granzyme and perforin that directly kill cancer cells.CD4+T helps other T cells function properly and memory T cells participate in secondary immune response.
T lymphocytes (T cells) are mainly involved in adaptive immune response; miRNA is involved in the regulation of T cell development, maturation, differentiation, and function18,19.In this review, recent findings and current understanding of the function of miRNAs in T cells are presented.Further studies should be conducted to elucidate the mechanism by which miRNAs regulate T cells in the context of immune therapy for malignant tumors and cancer immunosuppressive environments.
MiRNAs and T cell development
miRNAs exhibit dynamic changes during the development of hematopoietic stem cells.The expression level of miRNAs is highly related to the degree of T cell differentiation and development20.For example, miR-125b is expressed at a higher extent in lymphoid stem cells than in myeloid stem cells and hematopoietic stem cells.This high expression stimulates the development of lymphocyte lineage.MiR-125b is also involved in hematopoietic stem cell survival and functions in the maintenance of lymphoid balance21.In hematopoietic progenitor cells, high miR-181a expressions facilitates T and B cell development8,22.
Evidence has shown that miRNA is involved in T cell development in the thymus.Dicer, an RNaseIII-like enzyme necessary to generate short interfering RNAs and miRNAs, is required for CD8 T cell development23,24.MiRNA expression is dynamically regulated in distinct stages of thymic T cell development19; this result suggests that miRNAs participate in the regulation of T cell differentiation in the thymus.MiR-181a,which is specifically enriched at the CD4+CD8+DP stage of thymocyte development, suppresses the expression of Bcl-2,CD69, and T cell receptor (TCR); all of these molecules are important in positive selection.MiR-181a has also been shown to increase sensitivity to peptide antigens by downregulating multiple phosphatases25.These findings have indicated that miR-181a functions as an intrinsic “rheostat” in TCR signaling,which is very important in T cell development26.In addition to miR-181a, miR-150 is important in T cell development; the upregulation of miR-150 inhibits the expression of the target gene NOTCH327.
MiRNA is required for T cell activation,proliferation, and apoptosis
The activation of T cells depends on TCR and co-stimulatory molecules, such as CD28.T cells proliferate vigorously upon productive activation, leading to clonal expansion.Many miRNAs are involved in the regulation of T cell proliferation.After an external stimulation is detected, T cells greatly increase the expression of miR-214 and inhibit the expression of the target gene phosphatase and tensin homolog, which is deleted in chromosome ten (PTEN); as a result, T cell proliferation is enhanced18.In CD8+T cell activation, miR-130 and miR-301 are upregulated, resulting in a decreased CD69 expression through two miRNAs binding to the 3′-UTR of mRNA of CD6928.Grigorev et al.29demonstrated that high miR-155 and miR-221 expressions inhibit the expression of PIK3R1 as a co-target gene and further inhibits cell proliferation and cytokine production in CD4+T cells.A highly expressed miR-182 binds to Foxo1 of the 3′-UTR of mRNA and inhibits protein synthesis; this process results in an enhanced proliferation of T helper cells30.The function of miR-21 depends on the status of T cells.For instance, miR-21 regulates the survival of activated memory T cells and induces CCR-7 expression in activated naive T cells31.
The transcription factor c-Myc regulates cell proliferation,growth, and apoptosis.c-Myc also activates the expression of the miR-17~92 gene cluster.c-Myc is directly bound to this locus as observed in the results of chromatin immunoprecipitation assay.Two members of the miR-17~92 gene cluster, specifically miR-17-5p and miR-20a, negatively regulate the expression of E2F1, a known downstream gene of c-Myc.These findings have revealed a new mechanism by which c-Myc promotes E2F1 transcription and simultaneously limits translation; thus, cell proliferation is accurately controlled32.In Th1 cells, miR-19 and miR-17, as members of miR-17~92 gene cluster, target PTEN and cAMP-response element binding protein (CREB1), respectively; these miRNAs also participate in the immune response of Th1 cells by enhancing T cell proliferation, promoting cytokine production,and inhibiting apoptosis33,34.As CD8+T cells are stimulated by viral infection, miRNA profiling is instantaneously changed.Among these miRNAs, miR-17~92 cluster fails to regulate the expression levels of proteins with controlled effector and memory CD8+T cell differentiation35.In mice, high miR-17 to 92 expression in lymphocytes causes lymphoproliferative disease and autoimmunity; as a result, the mice died prematurely.Lymphocytes from these mice are also hyperpoliferative and resistant to apoptosis possibly because miR-17~92 suppresses the expression of the tumor suppressor PTEN and the proapoptotic protein Bim36.Another study has shown that miR-150 and miR-139 regulate perforin, eomesodermin, and IL-2Rα expression in the progression of CTL cell differentiation, which is involved in inflammation and antigen stimulation37.
Many miRNAs are involved in the regulation of T cell apoptosis.In CD4+T cells from patients with relapsing forms of multiple sclerosis, miR-15a and miR-16-1 are downregulated and the target-gene B-cell lymphoma 2 (Bcl-2) was upregulated;this result affects the progression of apoptosis38.Bim is a member of Bcl-2 family and participates in the mediation of lymphocyte apoptosis39.In patients with malignant lymphoma,glucocorticoid inhibits the expression of miR-17~92 gene cluster and this result is consistent with a high Bim expression.miR-17~92 overexpression reduces Bim expression levels and inhibits glucocorticoid-mediated apoptosis.By contrast, the knockdown of miR-17~92 increases Bim protein expression,thereby enhancing apoptosis40.However, the function of these miRNAs in T cells remains unclear.In another study, miR-122 is expressed in cutaneous T cell lymphoma (CTCL).In apoptotic CTCL cells triggered by various chemotherapeutic drugs, miR-122 is further upregulated.MiR-122 overexpression induces Akt activation and p53 inhibition, resulting in the resistance to chemotherapy-induced apoptosis.These data indicated that miR-122 amplifies the Akt/p53 anti-apoptosis pathway41.
MiRNA functions in the development of functional peripheral T cell subsets
Mature peripheral T cells consist mainly of regulatory T cells(Tregs), CD4, and CD8 T cells.These cells further differentiate into various functional subsets.The function of miRNAs in the development of divergent T cell subsets has been elucidated in several studies.
The miRNA expression in naive, effector, and memory CD8+T cells has been studied and dynamic changes in the miRNA pro file during peripheral T cell differentiation have been revealed.The downregulation of miRNAs has been observed in effector T cells compared with naive cells and memory T cells.In effector T cells, six miRNAs (let-7f, miR-15b, miR-142-5p, miR-150, miR-142-3p, and miR-16) are expressed at a low extent8.By contrast, a few miRNAs, such as miR-21, are highly expressed in effector T cells compared with memory and naive T cells.In antigen-stimulated CD8+T cells, miR-155, miR-21, and miR-146a are upregulated14.
Using an in vitro system in which activated CD8 T cells are driven by IL-2 or IL-15 function as either effector memory cells or central memory cells, we observed that numerous miRNAs, such as miR-150, miR-155, and the let-7 family, are associated with the development of these memory T cell subsets.In particular, miR-150 regulates the protein expression of Kv channel interacting protein 1 (KChiP1) in mouse central memory T cells42.These findings demonstrated the possible functions of these miRNAs in the further development of peripheral T cells.
T helper cells can be divided into Th1, Th2, Th17, and Th9 cells based on cytokine profiles; miRNAs are important in the differentiation and function of these T cell subsets.For instance,miR-142-5p is associated with CD4+CD25+T cell proliferation by binding to the 3′-UTR of the mRNA of GARP43.MiR-21 regulates Th1 and Th2 polarization and inflammatory response via the IL-2 and IFN-γ signaling pathways44.The targeted ablation of miR-21 in mice results in reduced lung eosinophilia after allergen challenge, thereby significantly increasing Th1 cytokine IFN-γ levels and IL-12 production by dendritic cells.Mice infected with Listeria monocytogenes or Mycobacterium bovis bacillus Calmette-Guérin (BCG) exhibit a downregulated miR-29 expression in IFN-γ-producing natural killer cells, CD4+T cells, and CD8+T cells.MiR-29 suppresses IFN-γ production by directly targeting IFN-γ, T-bet, and Eomes mRNA45,46.IL-23 also participates in Th17 responses.One study showed that miRNA let-7f inhibits the expression of IL-23 reporter in CD4+T cells47;this result indicated the function of these miRNAs in Th17 responses.MiR-125p is transfected into naive T cells, which terminate differentiation from naive T cells to effector cells48.
Treg cells are responsible for the induction of immune tolerance and immune homeostasis.In the expression pro file of miRNA from Treg cells, miR-24, miR-210, miR-95, and miR-145 are upregulated; by comparison, miR-24 and miR-210 negatively regulate FOXP3; miR-95 positively regulates FOXP3 via an indirect mechanism.In addition, miR-145 negatively regulates CTLA-4 expression49.Takahshi et al.50reported that miR-10a is highly expressed in Treg cells.MiR-10a is induced by retinoic acid and transforming growth factor-β (TGF-β), which target the transcriptional suppressor Bcl-6 and the co-suppressor Ncor2;as a result, the phenotypic conversion of Treg into follicular Thcells is decreased.Moreover, miR-10a limits the generation ofTh17 cells from the differentiation of Treg cells50.In stimulated Treg cells, miR-155 is upregulated; FOXOa3, the target of miR-155, negatively affects the Akt signaling pathway51.MiR-146 also inhibits the signal transducer and activator of transcription 1(STAT1) expression by controlling Th1 responses via Treg cellmediated regulation52.
MiRNA in cancer immunity
Type-1 T cells are important for the effective inhibition of tumor immune responses.In immune microenvironments, immune cells interact with cancer cells; with the passage of time, immune cells are stimulated by cancer cells and act against cancer cells by secreting small molecules.MiRNAs are a large family of small regulatory RNAs that function at a post-transcriptional level regulated by different processes of cell functions, including immune system regulation.miR-17~92 are downregulated in tumor microenvironments with specific T cells compared with normal T cells53.MiRNAs also regulate the co-stimulation of expressed molecules, such as intercellular adhesion molecule-1(ICAM-1)54, B7-H155, B7-H356, and cytokine57, which co-exist in tumor microenvironments.MiRNAs affect anti-tumor immunity by balancing the development, differentiation, and function of immune cells as well as the secretion of cytokines in local tumor microenvironments (Table 1).
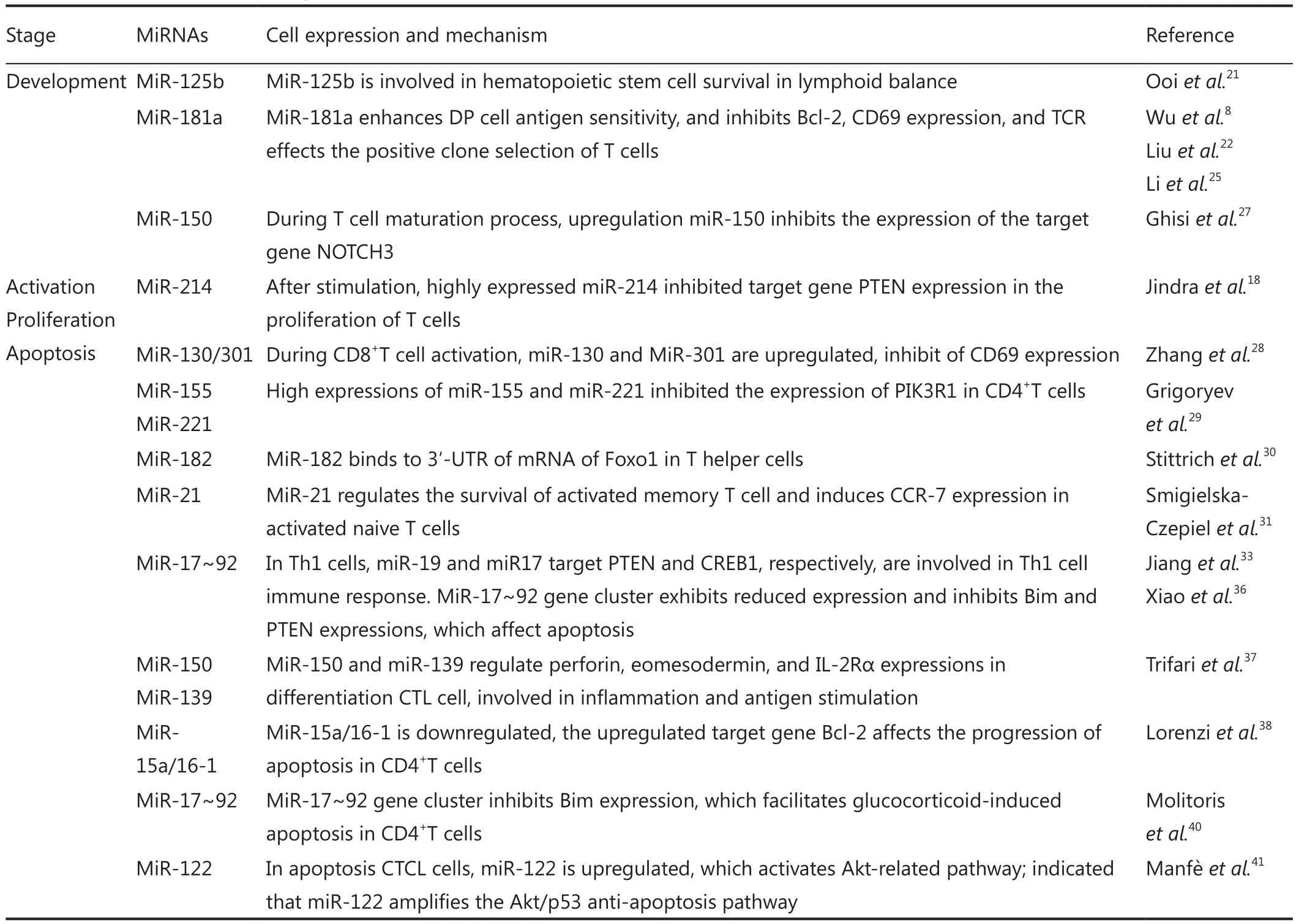
Table 1 Mechanism of miRNA-regulated T cells
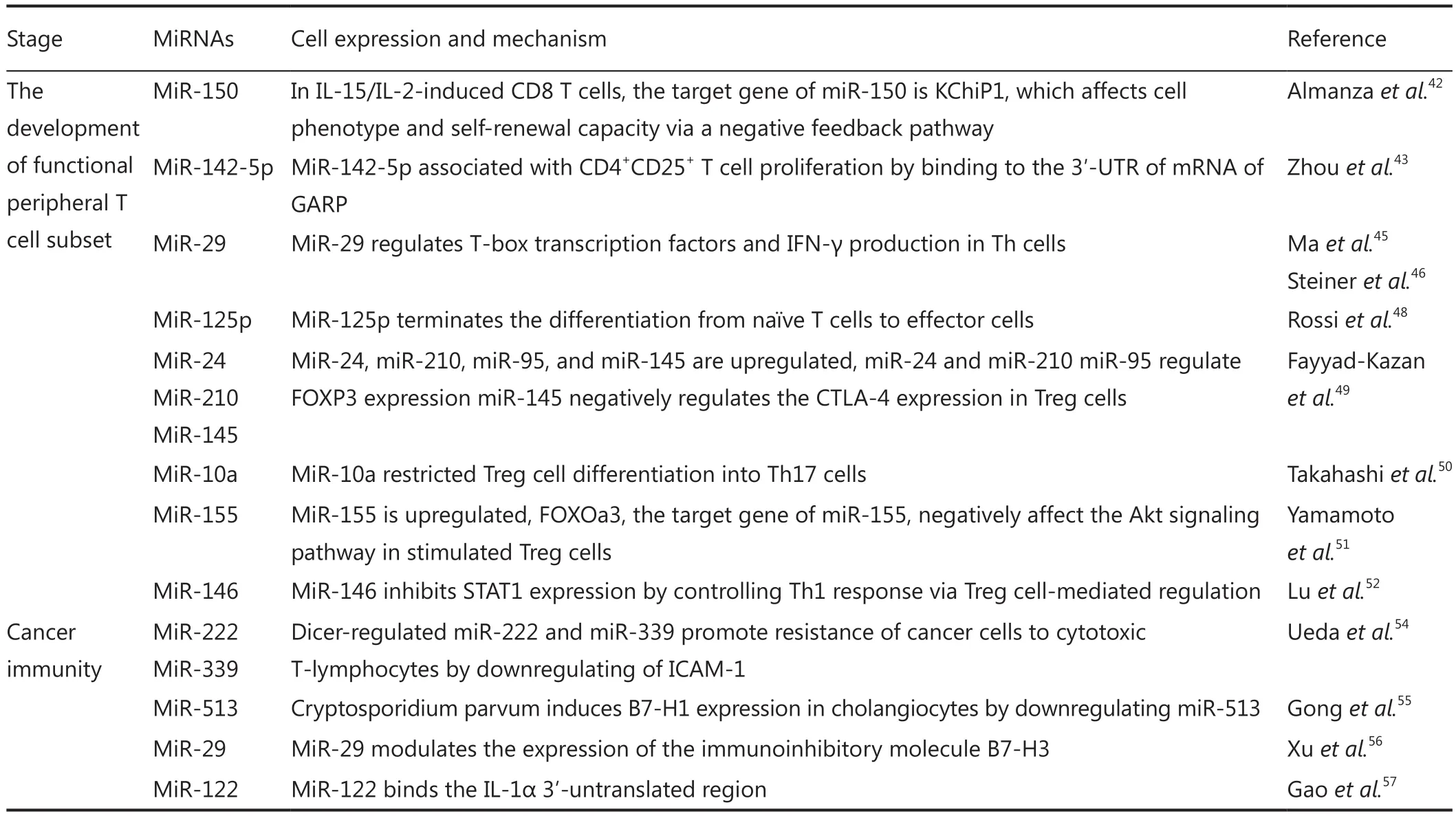
Table 1 (continued)
Conclusion
Increasing evidence suggests that miRNAs are important in the progression, development, and formation of immune systems.Therefore, miRNA regulatory networks should be further investigated in the context of disease settings to help elucidate the function of miRNAs in tumor microenvironments and inflammatory environments.Studies have focused on the mechanism of miRNA regulation.MiRNA should be engineered and applied in tumor microenvironments to inhibit oncogenes or suppress gene expression.In this way, miRNAs can function more effectively and accurately.Understanding the mechanism of T cell regulation by miRNAs, we may develop new therapies.Studies on engineering miRNAs have provided valuable information regarding the methods by which we could improve anti-tumor activity against solid tumors, as well as immune,autoimmune, and lymphatic diseases.
This study was supported by the National Natural Science Foundation of China (Grant Nos.81171653 and 30972703),Natural Science Foundation of Jiangsu Province (Grant Nos.BK2011246 and BK2011247), and Jiangsu Provincial Innovation Award BC2012093 by the Bureau of Science and Technology of Jiangsu Province.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Lopez-Camarillo C, Marchat LA, Arechaga-Ocampo E, Perez-Plasencia C, Del Moral-Hernandez O, Castaneda-Ortiz EJ, et al.MetastamiRs: Non-Coding MicroRNAs Driving Cancer Invasion and Metastasis.Int J Mol Sci 2012;13:1347-1379.
2.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD.Cancer immunoediting: from immunosurveillance to tumor escape.Nat Immunol 2002;3:991-998.
3.Zhang HG, Zhuang X, Sun D, Liu Y, Xiang X, Grizzle WE.Exosomes and immune surveillance of neoplastic lesions: a review.Biotech Histochem 2012;87:161-168.
4.Okada H, Kohanbash G, Lotze MT.MicroRNAs in immune regulation--opportunities for cancer immunotherapy.Int J Biochem Cell Biol 2010;42:1256-1261.
5.Holmstr?m K, Pedersen AW, Claesson MH, Zocca MB, Jensen SS.Identification of a microRNA signature in dendritic cell vaccines for cancer immunotherapy.Hum Immunol 2010;71:67-73.
6.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, et al.Identification of hundreds of conserved and nonconserved human microRNAs.Nat Genet 2005;37:766-770.
7.Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E.Phylogenetic shadowing and computational identification of human microRNA genes.Cell 2005;120:21-24.
8.Wu H, Neilson JR, Kumar P, Manocha M, Shankar P, Sharp PA,et al.miRNA pro filing of naive, effector and memory CD8 T cells.PLoS One 2007;2:e1020.
9.Bushati N, Cohen SM.microRNA functions.Annu Rev Cell Dev Biol 2007;23:175-205.
10.Xiao C, Rajewsky K.MicroRNA control in the immune system:basic principles.Cell 2009;136:26-36.
11.Bartel DP.MicroRNAs: genomics, biogenesis, mechanism, and function.Cell 2004;116:281-297.
12.Banerjee D, Kwok A, Lin SY, Slack FJ.Developmental timing in C.elegans is regulated by kin-20 and tim-1, homologs of core circadian clock genes.Dev Cell 2005;8:287-295.
13.Starr TK, Jameson SC, Hogquist KA.Positive and negative selection of T cells.Annu Rev Immunol 2003;21:139-176.
14.Salaun B, Yamamoto T, Badran B, Tsunetsugu-Yokota Y, Roux A,Baitsch L, et al.Differentiation associated regulation of microRNA expression in vivo in human CD8+ T cell subsets.J Transl Med 2011;9:44.
15.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM,et al.Four functionally distinct populations of human effectormemory CD8+ T lymphocytes.J Immunol 2007;178:4112-4119.
16.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL,Murphy KM, et al.Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages.Nat Immunol 2005;6:1123-1132.
17.Baumjohann D, Ansel KM.MicroRNA-mediated regulation of T helper cell differentiation and plasticity.Nat Rev Immunol 2013;13:666-678.
18.Jindra PT, Bagley J, Godwin JG, Iacomini J.Costimulationdependent expression of microRNA-214 increases the ability of T cells to proliferate by targeting Pten.J Immunol 2010;185:990-997.
19.Neilson JR, Zheng GX, Burge CB, Sharp PA.Dynamic regulation of miRNA expression in ordered stages of cellular development.Genes Dev 2007;21:578-589.
20.Bi Y, Liu G, Yang R.MicroRNAs: novel regulators during the immune response.J Cell Physiol 2009;218:467-472.
21.Ooi AG, Sahoo D, Adorno M, Wang Y, Weissman IL, Park CY.MicroRNA-125b expands hematopoietic stem cells and enriches for the lymphoid-balanced and lymphoid-biased subsets.Proc Natl Acad Sci U S A 2010;107:21505-21510.
22.Liu G, Min H, Yue S, Chen CZ.Pre-miRNA loop nucleotides control the distinct activities of mir-181a-1 and mir-181c in early T cell development.PLoS One 2008;3:e3592.
23.Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A,Rajewsky K.Aberrant T cell differentiation in the absence of Dicer.J Exp Med 2005;202:261-269.
24.Cobb BS, Hertweck A, Smith J, O’Connor E, Graf D, Cook T, et al.A role for Dicer in immune regulation.J Exp Med 2006;203:2519-2527.
25.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, et al.miR-181a is an intrinsic modulator of T cell sensitivity and selection.Cell 2007;129:147-161.
26.Fragoso R, Mao T, Wang S, Schaffert S, Gong X, Yue S, et al.Modulating the strength and threshold of NOTCH oncogenic signals by mir-181a-1/b-1.PLoS Genet 2012;8:e1002855.
27.Ghisi M, Corradin A, Basso K, Frasson C, Sera fin V, Mukherjee S, et al.Modulation of microRNA expression in human T-cell development: targeting of NOTCH3 by miR-150.Blood 2011;117:7053-7062.
28.Zhang N, Bevan MJ.Dicer controls CD8+ T-cell activation,migration, and survival.Proc Natl Acad Sci U S A 2010;107:21629-21634.
29.Grigoryev YA, Kurian SM, Hart T, Nakorchevsky AA, Chen C,Campbell D, et al.MicroRNA regulation of molecular networks mapped by global microRNA, mRNA, and protein expression in activated T lymphocytes.J Immunol 2011;187:2233-2243.
30.Stittrich AB, Haftmann C, Sgouroudis E, Kuhl AA, Hegazy AN,Panse I, et al.The microRNA miR-182 is induced by IL-2 and promotes clonal expansion of activated helper T lymphocytes.Nat Immunol 2010;11:1057-1062.
31.Smigielska-Czepiel K, van den Berg A, Jellema P, Slezak-Prochazka I, Maat H, van den Bos H, et al.Dual Role of miR-21 in CD4+T-Cells: Activation-Induced miR-21 Supports Survival of Memory T-Cells and Regulates CCR7 Expression in Naive T-Cells.PLoS One 2013;8:e76217.
32.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT.c-Myc-regulated microRNAs modulate E2F1 expression.Nature 2005;435:839-843.
33.Jiang S, Li C, Olive V, Lykken E, Feng F, Sevilla J, et al.Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation.Blood 2011;118:5487-5497.
34.Lykken EA, Li QJ.microRNAs at the regulatory frontier: an investigation into how microRNAs impact the development and effector functions of CD4 T cells.Immunol Res 2011;49:87-96.
35.Wu T, Wieland A, Araki K, Davis CW, Ye L, Hale JS, et al.Temporal expression of microRNA cluster miR-17-92 regulates effector and memory CD8+ T-cell differentiation.Proc Natl Acad Sci U S A 2012;109:9965-9970.
36.Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J,et al.Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes.Nat Immunol 2008;9:405-414.
37.Trifari S, Pipkin ME, Bandukwala HS, Aij? T, Bassein J, Chen R, et al.MicroRNA-directed program of cytotoxic CD8+ T-cell differentiation.Proc Natl Acad Sci U S A 2013;110:18608-18613.
38.Lorenzi JC, Brum DG, Zanette DL, de Paula Alves Souza A,Barbuzano FG, Dos Santos AC, et al.miR-15a and 16-1 Are Downregulated in CD4(+) T Cells of Multiple Sclerosis Relapsing Patients.Int J Neurosci 2012;122:466-71.
39.Marrack P, Kappler J.Control of T cell viability.Annu Rev Immunol 2004;22:765-787.
40.Molitoris JK, McColl KS, Distelhorst CW.Glucocorticoidmediated repression of the oncogenic microRNA cluster miR-17~92 contributes to the induction of Bim and initiation of apoptosis.Mol Endocrinol 2011;25:409-420.
41.Manfè V, Biskup E, Rosbjerg A, Kamstrup M, Skov AG, Lerche CM, et al.miR-122 regulates p53/Akt signalling and the chemotherapy-induced apoptosis in cutaneous T-cell lymphoma.PLoS One 2012;7:e29541.
42.Almanza G, Fernandez A, Volinia S, Cortez-Gonzalez X, Croce CM, Zanetti M.Selected microRNAs de fine cell fate determination of murine central memory CD8 T cells.PLoS One 2010;5:e11243.
43.Zhou Q, Haupt S, Prots I, Thümmler K, Kremmer E, Lipsky PE, et al.miR-142-3p is involved in CD25+ CD4 T cell proliferation by targeting the expression of glycoprotein A repetitions predominant.J Immunol 2013;190:6579-6588.
44.Lu TX, Hartner J, Lim EJ, Fabry V, Mingler MK, Cole ET, et al.MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization,and the severity of delayed-type hypersensitivity.J Immunol 2011;187:3362-3373.
45.Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, et al.The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma.Nat Immunol 2011;12:861-869.
46.Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CD, et al.MicroRNA-29 regulates T-box transcription factors and interferongamma production in helper T cells.Immunity 2011;35:169-181.
47.Li Z, Wu F, Brant SR, Kwon JH.IL-23 receptor regulation by Let-7f in human CD4+ memory T cells.J Immunol 2011;186:6182-6190.
48.Rossi RL, Rossetti G, Wenandy L, Curti S, Ripamonti A, Bonnal RJ, et al.Distinct microRNA signatures in human lymphocyte subsets and enforcement of the naive state in CD4+ T cells by the microRNA miR-125b.Nat Immunol 2011;12:796-803.
49.Fayyad-Kazan H, Rouas R, Fayyad-Kazan M, Badran R, El Zein N, Lewalle P, et al.MicroRNA pro file of circulating CD4-positive regulatory T cells in human adults and impact of differentially expressed microRNAs on expression of two genes essential to their function.J Biol Chem 2012;287:9910-9922.
50.Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciume G,Muljo SA, et al.TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells.Nat Immunol 2012;13:587-595.
51.Yamamoto M, Kondo E, Takeuchi M, Harashima A, Otani T, Tsuji-Takayama K, et al.miR-155, a Modulator of FOXO3a Protein Expression, Is Underexpressed and Cannot Be Upregulated by Stimulation of HOZOT, a Line of Multifunctional Treg.PLoS One 2011;6:e16841.
52.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, et al.Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses.Cell 2010;142:914-929.
53.Sasaki K, Kohanbash G, Hoji A, Ueda R, McDonald HA,Reinhart TA, et al.miR-17-92 expression in differentiated T cells -implications for cancer immunotherapy.J Transl Med 2010;8:17.
54.Ueda R, Kohanbash G, Sasaki K, Fujita M, Zhu X, Kastenhuber ER, et al.Dicer-regulated microRNAs 222 and 339 promote resistance of cancer cells to cytotoxic T-lymphocytes by downregulation of ICAM-1.Proc Natl Acad Sci U S A 2009;106:10746-10751.
55.Gong AY, Zhou R, Hu G, Liu J, Sosnowska D, Drescher KM,et al.Cryptosporidium parvum induces B7-H1 expression in cholangiocytes by down-regulating microRNA-513.J Infect Dis 2010;201:160-169.
56.Xu H, Cheung IY, Guo HF, Cheung NK.MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3:potential implications for immune based therapy of human solid tumors.Cancer Res 2009;69:6275-6281.
57.Gao Y, He Y, Ding J, Wu K, Hu B, Liu Y, et al.An insertion/deletion polymorphism at miRNA-122-binding site in the interleukin-1alpha 3’ untranslated region confers risk for hepatocellular carcinoma.Carcinogenesis 2009;30:2064-2069.
 Cancer Biology & Medicine2013年3期
Cancer Biology & Medicine2013年3期
- Cancer Biology & Medicine的其它文章
- Comparative study of CEA and CA19-9 in esophageal, gastric and colon cancers individually and in combination (ROC curve analysis)
- Effect of chemotherapy on autoimmune hepatitis in thymoma:a case report and literature review
- Prognositic factors and clinicopathologic characteristics of small gastrointestinal stromal tumor of the stomach: a retrospective analysis of 31 cases in one center
- Effect of flupirtine on the growth and viability of U373 malignant glioma cells
- Identification of differentially expressed long non-coding RNAs in human ovarian cancer cells with different metastatic potentials
- Advances in circulating microRNAs as diagnostic and prognostic markers for ovarian cancer
