Matrix metalloproteinase gene expressions might be oxidative stress targets in gastric cancer cell lines
Salih Gencer, Anil Cebeci, Meliha Burcu Irmak-Yazicioglu
1Department of Genetics and Bioengineering, Faculty of Engineering, Fatih University, Istanbul 34500, Turkey;2Department of Molecular Biology and Genetics, Faculty of Engineering and Natural Sciences, Uskudar University, Istanbul 34662, Turkey;3Department of Molecular Biology and Genetics, Faculty of Arts and Sciences, Halic University, Istanbul 34381, Turkey
Matrix metalloproteinase gene expressions might be oxidative stress targets in gastric cancer cell lines
Salih Gencer1,2, Anil Cebeci3, Meliha Burcu Irmak-Yazicioglu3
1Department of Genetics and Bioengineering, Faculty of Engineering, Fatih University, Istanbul 34500, Turkey;2Department of Molecular Biology and Genetics, Faculty of Engineering and Natural Sciences, Uskudar University, Istanbul 34662, Turkey;3Department of Molecular Biology and Genetics, Faculty of Arts and Sciences, Halic University, Istanbul 34381, Turkey
Corresponding to:Meliha Burcu Irmak-Yazicioglu, Assoc. Prof. Department of Molecular Biology and Genetics, Faculty of Arts and Sciences, Halic University, Siracevizler Str., No. 29 Bomonti, Istanbul 34381, Turkey. Email: burcuyazicioglu@halic.edu.tr.
Objective:Oxidative stress is linked to increased risk of gastric cancer and matrix metalloproteinases (MMPs) are important in the invasion and metastasis of gastric cancer. We aimed to analyze the effect of the accumulation of oxidative stress in the gastric cancer MKN-45 and 23132/87 cells following hydrogen peroxide (H2O2) exposure on the expression patterns ofMMP-1,MMP-3,MMP-7,MMP-9,MMP-10,MMP-11,MMP-12,MMP-14,MMP-15,MMP-17,MMP-23,MMP-28, and β-catenin genes.
Methods:The mRNA transcripts in the cells were determined by RT-PCR. Following H2O2exposure, oxidative stress in the viable cells was analyzed by 2',7'-dichlorofluorescein diacetate (DCFH-DA). Caffeic acid phenethyl ester (CAPE) was used to eliminate oxidative stress and the consequence of H2O2exposure and its removal on the expressions of the genes were evaluated by quantitative real-time PCR.
Results:The expressions ofMMP-1,MMP-7,MMP-14,MMP-15,MMP-17and β-catenin in MKN-45 cells and only the expression ofMMP-15in 23132/87 cells were increased. Removal of the oxidative stress resulted in decrease in the expressions ofMMPgenes of which the expressions were increased after H2O2exposure. β-catenin, a transcription factor for many genes includingMMPs, also displayed decreased levels of expression in both of the cell lines following CAPE treatment.
Conclusions:Our data suggest that there is a remarkable link between the accumulation of oxidative stress and the increased expressions ofMMPgenes in the gastric cancer cells and MMPs should be considered as potential targets of therapy in gastric cancers due to its continuous exposure to oxidative stress.
Oxidative stress; hydrogen peroxide (H2O2); matrix metalloproteinases (MMPs); expression; caffeic acid phenethyl ester (CAPE)
Scan to your mobile device or view this article at:http://www.thecjcr.org/article/view/2202/3062
Introduction
To live in homeostasis, every single organism must control the regulatory mechanism of itself against the changing conditions of the environment. From bacteria to mammals, oxidative stress has devastating effects which are the protein dysfunction, the lipid peroxidation, the breakdown of DNA, the change of the genetic code via mutations, and finally apoptosis (1,2).
Oxidative stress can trigger many cell signaling pathways to protect the normal cell life. Low levels of oxidative stress activate the transcription of genes encoding proteins that participate in the defense against oxidative injuries, oxidative damage repair mechanisms, and apoptosis (3). Nuclear accumulation of Nrf-2 and NF-κB causes transcriptional activation of antioxidant enzymes including superoxide dismutases, glutathione peroxidases and thioredoxin, respectively (3-6). If these first defense attempts are not sufficient to prevent oxidative stress-induced DNA damage, several other signaling processes such as base excision repair (7), cell cycle arrest at G1-S transition,S phase, and G2-M transitions (8) serve as protective mechanisms. In MCF-7 breast cancer cells, oxidative stress causes the expressions ofCYGB, FOXM1, NOX5, NUDT1andSEPP1genes which can be affected differentially. Especially, the expression ofFOXM1may suppress cancer development and increases the effects of anticarcinogenic agents (9). In MDA-MB-231 breast cancer cells, oxidative stress responses of the cells are increased by NRP/B, a nuclear matrix protein, via the Nrf2 pathway. Nrf2 pathway associates with antioxidant response element (ARE) sourced detoxifying and antioxidant genes (10). Oxidative stress also regulates the role of β-catenin which is a key element of canonical Wnt pathway through binding and activating T cell factor (TCF) transcription factor. Under oxidative stress, the transcriptional activity of β-catenin/TCF complex is inhibited (11,12). FOXO, another transcription factor that is modulated by β-catenin, interacts with β-catenin in increased oxidative stress resulting with increased expression of anti-oxidant genes. FOXO can also inhibit the cell cycle progress under this condition because of its regulator role in G1 transition of the cell cycle (12). Additionally, HIF-1 and HIF-2, transcription factors that are responsible for the response of cells to hypoxia, directly bind to β-catenin and regulate the adaptation of cells to hypoxia (13).
The balance between pro-oxidants and antioxidants determines the extent of oxidative damage induced by reactive oxygen species (ROS). The shift from antioxidants to pro-oxidants in the tissue has been linked to increased risk of cancer (14,15). Chronic inflammation as a consequence of persistent oxidative stress has been linked to various steps involved in carcinogenesis, including cellular transformation, promotion, survival, proliferation, invasion, angiogenesis, and metastasis (16,17). Our insight in the molecular pathways involved in these biological responses to different oxygen radicals is still incomplete.
Matrix metalloproteinases (MMPs) are zinc-dependent proteins that are responsible for degrading extracellular matrix. Due to their capabilities, they can play important roles in growth, development and pathological processes. In carcinogenesis, they may provide suitable growth, invasion and finally metastasis conditions for tumor cells. Different MMP types can take action in different stages of carcinogenesis. These all depend on the cell environment and the needs of tumor progression (18).
Gastric cancer is the seventh most common cancer and the second most common cause of cancer-related death worldwide (19). The gastric epithelium is continuously exposed to toxic ROS within the gastric lumen due to ingested food and cigarette smoke and inflammation due toHelicobacter pyloriinfection. The dynamic balance between cell proliferation and apoptosis is essential for maintaining mucosal homeostasis. Decreased apoptosis as well as increased proliferation may favor the carcinogenic process. Prolonged survival of abnormal cells can support the accumulation of sequential genetic mutations, changes in gene expression profiles and protein structure and function which can result in tumor promotion (20-24). Consequently, the link between oxidative stress and gastric cancer cannot be ignored. However, the molecular mechanisms behind it are still not clear.
In this study, the changes in the expression levels ofMMPgenes were analyzed following H2O2exposure of gastric carcinoma MKN-45 and 23132/87 cell lines to understand whether a relation exists between oxidative stress formation in gastric cancer cells and the expressions ofMMPgenes.
Materials and methods
Cell culture
Human gastric adenocarcinoma cell lines, MKN-45 (DSMZ ACC409) and 23132/87 (DSMZ ACC201), were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) (Braunschweig, Germany). The cells were cultured in a standard RPMI-1640 (Biochrom, Berlin, Germany) medium supplemented with 10% (V/V) fetal bovine serum (FBS) and 1% (V/V) penicillin and streptomysin (10,000 μg/mL, Biochrom) in a humidified chamber at 37 ℃ in the presence of 5% CO2.
H2O2treatment of cell lines
The cells were plated at a density of 3×103-5×103cells per well and cultured in the standard medium overnight. The medium was aspirated and washed with phosphate buffered saline (PBS); the cells were starved in the culture medium supplemented with 0.01% FBS overnight and they were treated with indicated concentrations of H2O2ranging from 50-200 μmol/L for indicated time points. The control cells were only starved and were not exposed to H2O2. The morphological changes were analyzed under microscope (Axiovert 40 CFL, Zeiss, Jena, Germany).
Determination of 8-hydroxydeoxyguanosine (8-OHdG) in cell lines
MKN-45 and 23132/87 cell lines that were exposed to H2O2ranging from 50 to 200 μmol/L for 24 h were fixed with 4% paraformaldehyde for 15 min at room temperature. After the fixation, the presence of 8-OHdG in the cells was determined using DakoCytomation LSAB2 System-HRP kit (Dako, Hamburg, Germany). Mouse monoclonal 8-OHdG antibody (Cosmobio, Tokyo, Japan) was used as primary antibody. The sections were treated for 30 min at room temperature first with biotinylated goat antirabbit and goat anti-mouse IgG (Cosmobio) and then with streptavidin-horseradish peroxidase (HRP) solution. Between incubations, the sections were washed with 1× PBS (pH 7.4). 3-3'-diaminobenzidine tetrahydrochloride (DAB) (Sigma Chemical Co., St. Louis, Mo., USA) was used as HRP substrate. The sections were incubated with substrate-chromogen solution for 5 min and counterstaining was performed with Mayer’s hematoxylin to visualize the nucleus (Zeiss, Axiovert 40 CFL).
Reverse transcription-polymerase chain reaction (RT-PCR)
mRNA transcripts encodingMMP-1,MMP-3,MMP-7,MMP-9,MMP-10,MMP-11,MMP-12,MMP-14,MMP-15,MMP-17,MMP-23,MMP-28, β-catenin, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) genes were determined by RT-PCR. Samples were homogenized to break them up. RNA was extracted from the cell lines using NucleoSpin RNA II kit (Macherey-Nagel, Oensingen, Switzerland). RNA concentration and purity of each extract were determined by A260/A280absorptions using Shimadzu UV-1202 spectrophotometer. RNA extracts were stored at –80 ℃ for long-term use. cDNA was synthesized using oligo(dT)18primer according to the manual of RevertAidTMFirst Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania). Primers for amplification of selectedMMPs, β-catenin, andGAPDHand the PCR conditions are shown inTable 1. To determine the optimum number of cycles required for the amplification of these genes, an aliquot of first strand cDNA was amplified with the respective primers using an increasing number of PCR cycles (20-35). Initial denaturation was performed at 94 ℃ for 4 min. The subsequent cycling programs consisted of denaturation at 95 ℃ for 30 s, annealing for 30 s (annealing temperatures, seeTable 1), and extension at 72 ℃ for 30 s, followed by a final extension at 72 ℃ for 5 min. The amplified products were separated on a 2% agarose gel, stained with ethidium bromide, and photographed using Gel Doc 2000 imaging system (Bio-Rad, Hercules, CA). PCR reactions in which the first strand cDNA was omitted served as negative controls. To avoid technical error, each PCR experiment was repeated three times.
Determination of oxidative stress in cell lines
Oxidative stress in the cell lines was assessed using an oxidative stress marker, 2',7'-dichlorofluorescein diacetate (DCFHDA) (25). The non-fluorescent DCFH-DA (Sigma) is a cellpermeable compound that can enter into the cells, where it is deacetylated and entrapped as 2',7'-dichlorofluorescein (DCFH) of which by ROS produces a highly fluorescent product, DCF, which can be visualized under a fluorescent microscope. DCFH-DA is freshly prepared in 10 mmol/L HEPES (pH 7.5), 10 mmol/L glucose and 1 μmol/L DCFDA (dissolved in methanol) in PBS. MKN-45 and 23132/87 cells were treated with 200 μmol/L H2O2for 24 h and after this time point, the medium was aspirated, and the cells were washed two times with PBS, and incubated with DCFH-DA solution at 37 ℃ for 15 min, and then were washed two times with PBS and observed under fluorescent microscope (Axio-skop, Zeiss, Jena, Germany). The live cells that were under oxidative stress were counted in five 20× fields per culture (typically 70-100 cells/20× field), and the percentage of the cells under oxidative stress in each culture was calculated.
Removal of stress with caffeic acid phenethyl ester (CAPE) in cell lines
The generated oxidative stress with 200 μmol/L H2O2was eliminated with CAPE. Both of the cell lines were grown to 70% confluency in RPMI medium supplemented with 10% FBS. Three hours after the addition of 3 μg/mL CAPE (Sigma) to the growth medium, 200 μmol/L H2O2was applied to the cells. After 24 h of incubation of the cells in CO2incubator, the presence of oxidative stress in the live cells was demonstrated using DCFH as mentioned above. CAPE-untreated controls with only 200 μmol/L H2O2exposure were also used in the experiment.
Quantitative real-time PCR
Total RNA was isolated and cDNA was synthesized from the cell lines using RNA extraction kit (NucleoSpin RNA II, Macherey-Nagel) and cDNA synthesis kit (RevertAid?First Strand cDNA Synthesis Kit, Fermentas), respectively, according to the manufacturer’s instructions. Associated genes, their primers and annealing temperatures are listedinTable 1. As an internal control, theGAPDHmRNA was also amplified in PCR reactions. The reactions were carried out with 2 μL cDNA template in a total volume of 25 μL, containing 1× iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) with primers for whole genes in a Rotor-Gene real-time PCR instrument (Corbett Research, Sydney, Australia). After initial denaturation at 95 ℃for 10 min, 40 cycles of denaturation at 95 ℃ for 10 s, annealing at different temperatures from 55 to 63 ℃(seeTable 1) for 25 s and extension at 72 ℃ for 25 s were carried out. Finally, melting analysis was performed in the temperature range of 55 to 95 ℃ to verify product homogeneity. Real-time PCR reactions were carried out in triplicate for each sample as a technical replicate. Each cDNA sample was tested in three different reactions with three technical replicates and negative controls. Three biological replications have been performed for each transcript in order to determine whether there are significant differences in the expressions at the different time points.
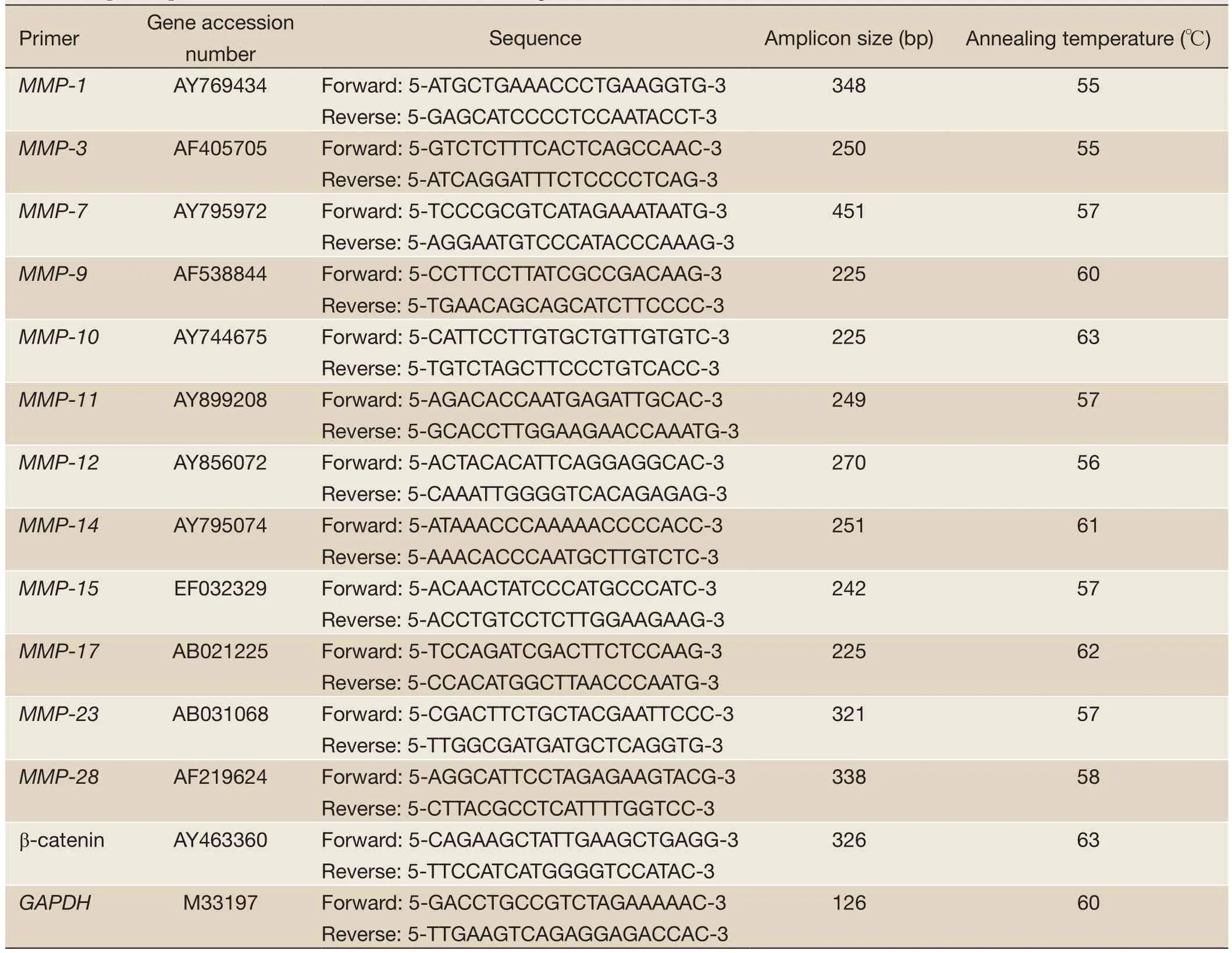
Table 1 Specific primers forMMP, β-catenin andGAPDHgenes
Statistical analysis
Each of the data is the mean of three independent values and all data are expressed asx±s. Student’st-test is used in order to assess whether the means of mRNA expression rates of untreated control cells and treated cells are statistically different from each other by using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
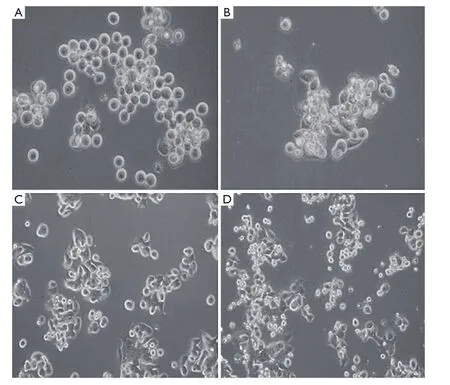
Figure 1 The inverted light microscope views of morphological changes in MKN-45 and 23132/87 cell lines treated with 200 μmol/L H2O2after 24 h (200×). A. control MKN-45; B. MKN-45 with 200 μmol/L H2O2treatment; C. control 23132/87; D. 23132/87 with 200 μmol/L H2O2treatment
Results
Morphological changes of cell lines
Gastric cancer cell lines, MKN-45 and 23132/87, exhibited a remarkable change in their morphologies and loss of surface attachment following H2O2exposure when they were compared with their H2O2-untreated controls as shown by inverted light microscopic views with the presence of 8-OHdG (data not shown). The unexposed control cells exhibited distinctive cell shape having regular plasma membrane boundaries with apparent cytoplasm and nucleus whereas H2O2exposure resulted in irregular cell membrane with nuclear and cytoplasmic shrinking, and chromatin condensation in the cell lines (Figure 1).
Determination of MMP expressions
MKN-45 and 23132/87 cell lines showed different expression patterns ofMMPgenes (Table 2).MMP-1(26),MMP-7(26),MMP-10,MMP-14andMMP-17genes were only expressed in MKN-45 cells, whileMMP-11,MMP-15(26),MMP-23andMMP-28genes were expressed in both of the cell lines. On the other hand,MMP-3(26),MMP-9andMMP-12gene expressions were determined in neither of them. Moreover, β-catenin expression was positive in both of the cell lines (Figure 2).
Effects of H2O2exposure on expressions of MMP genes
Among the fourMMPgenes that were expressed in 23132/87 cells, only the expression ofMMP-15gene was determined to be increased following H2O2exposure. However, in MKN-45 cells, there was an increase in the expressions ofMMP-1,MMP-7,MMP-14,MMP-15,MMP-17, and β-catenin (Table 3). As seen inFigure 3, 12 h exposure to H2O2resulted in a more increase in theexpression ofMMP-14gene compared to 24 h exposure to H2O2. The same pattern was observed forMMP-7gene as we have previously reported (27). Conversely,MMP-1,MMP-15andMMP-17exhibited more expression when MKN-45 cells were exposed to H2O2for 24 h. β-catenin expression was also affected positively by the application of H2O2to MKN-45 cells for 12 and 24 h.
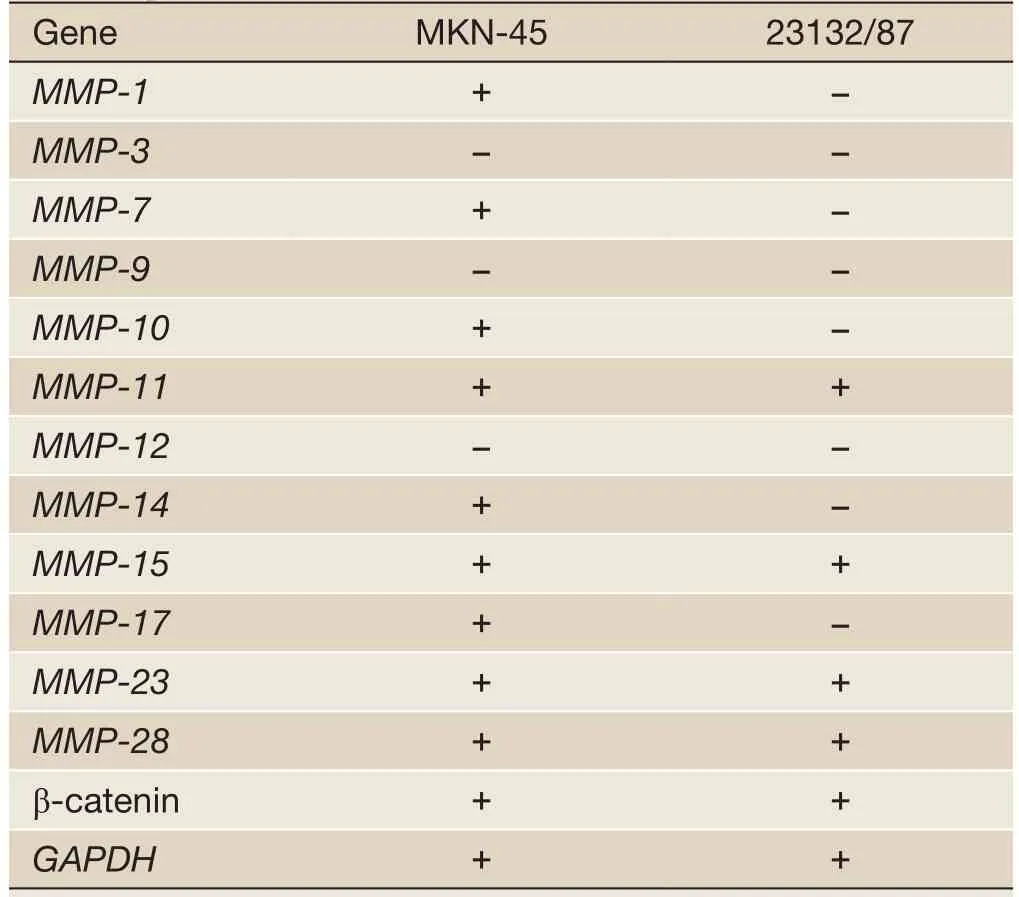
Table 2 Expression profiles ofMMPand β-cateningenes at the transcription level in MKN-45 and 23132/87 cell lines
β-catenin expression was significantly 0.75 (80%), 1.60 and 1.45 fold increased (P<0.05) after 12 h of H2O2exposure while it was 1.80, 1.45 and 0.90 (90%) fold increased significantly (P<0.05) after 24 h of oxidative stress treatment at 50, 100 and 200 μmol/L H2O2concentrations, respectively, compared with the untreated control cells. Following 12 h of H2O2application,MMP-14displayed a significant up-regulation of 1.40, 4.40 and 2.03 fold (P<0.05) and 24 h of H2O2application 2.45, 1.70 and 0.80 (80%) fold significant increase (P<0.05) at 50, 100 and 200 μmol/L H2O2concentrations, respectively, compared with the untreated control cells. The analysis of theMMP-14geneexpression gave parallel patterns of expression profiles with β-catenin. There was a 0.76 (76%), 1.69 and 1.35 fold significant increase (P<0.05) after 12 h H2O2exposure and 1.82, 1.33 and 0.92 (92%) fold significant increase (P<0.05) after 24 h H2O2treatment at 50, 100 and 200 μmol/L H2O2concentrations, respectively, compared with the control cells inMMP-14gene (Figure 3). Likewise,MMP-7gene showed a similar pattern of increase with different values at the same order of the concentrations (27).
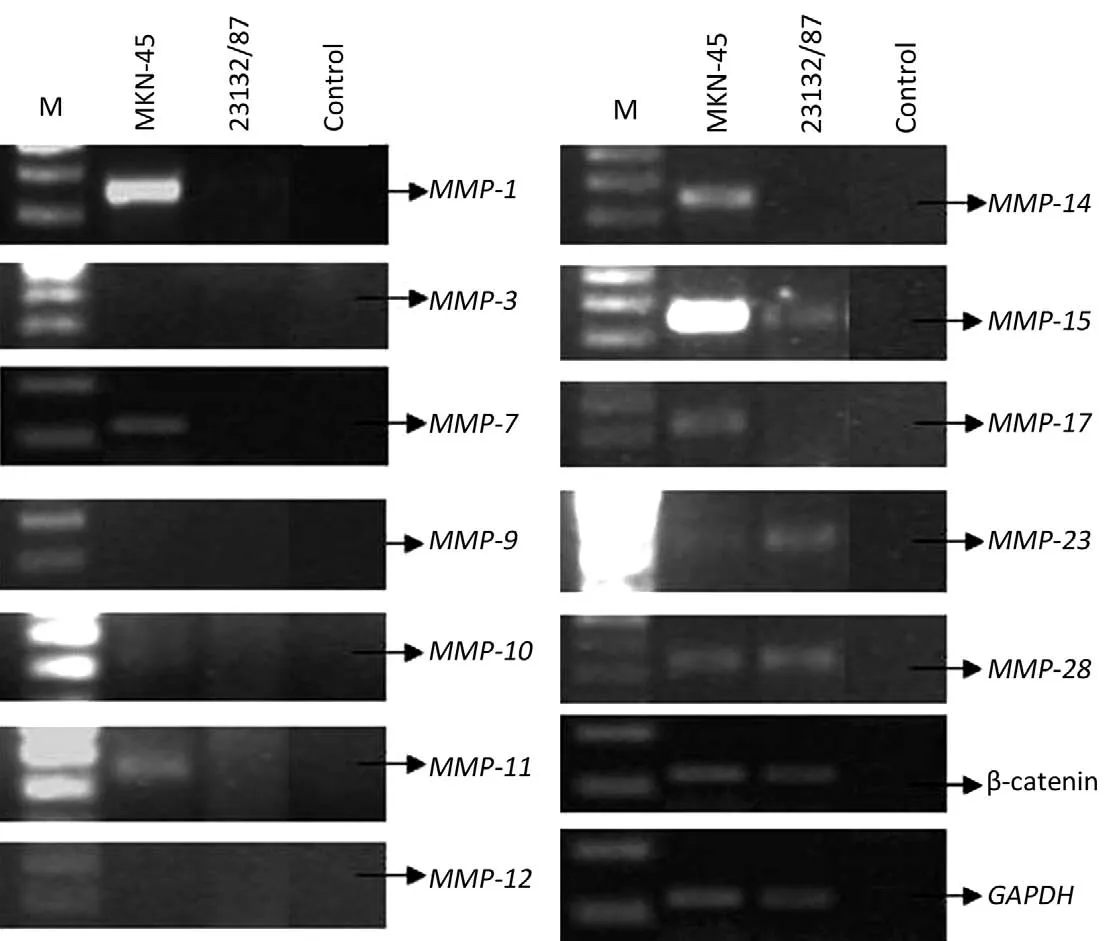
Figure 2 RT-PCR analysis of severalMMPgenes, β-catenin andGAPDHin MKN-45 and 23132/87 cell lines. M, 100 bp molecular weight marker
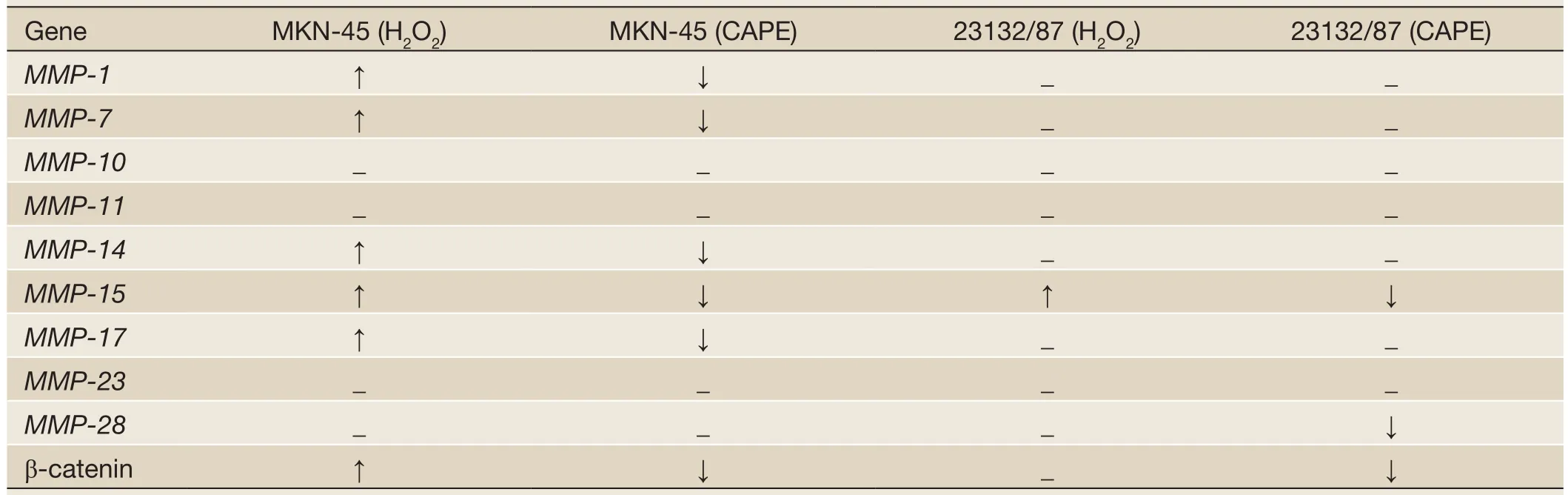
Table 3 The change of gene expression which intracellular oxidative stress is removed with CAPE in cell lines
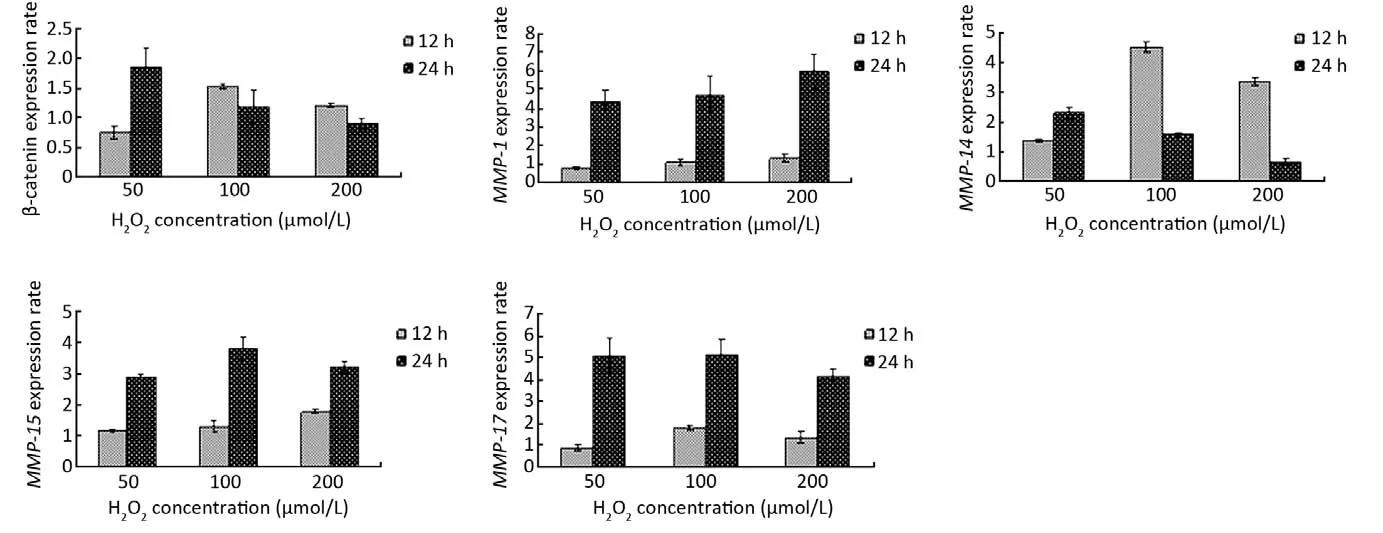
Figure 3 The expression rates of β-catenin,MMP-1,MMP-14, MMP-15andMMP-17genes in MKN-45 cells treated with H2O2compared to the untreated control cells at 12 and 24 h under indicated H2O2concentrations. Each of data is the average of three independent values and standard deviations are indicated above (σn-1) data values. All the up-regulations observed in the expressions were taken as statistically significant (P<0.05) as determined by studentt-test
The expression ofMMP-1gene in MKN-45 cells after 12 h of H2O2exposure was nearly similar at all the three concentrations, but 24 h exposure to H2O2caused a significant up-regulation of 4.6, 4.9 and 6.1 fold (P<0.05) at 50, 100 and 200 μmol/L concentrations, respectively. The same profile was observed in theMMP-15andMMP-17genes. Subsequent to 24 h H2O2exposure,MMP-15expression was significantly increased by 3.0, 3.9 and 3.4 fold (P<0.05) andMMP-17expression was significantly upregulated by 5.05, 5.10 and 4.30 fold (P<0.05) at 50, 100, and 200 μmol/L concentrations of H2O2, respectively (Figure 3).
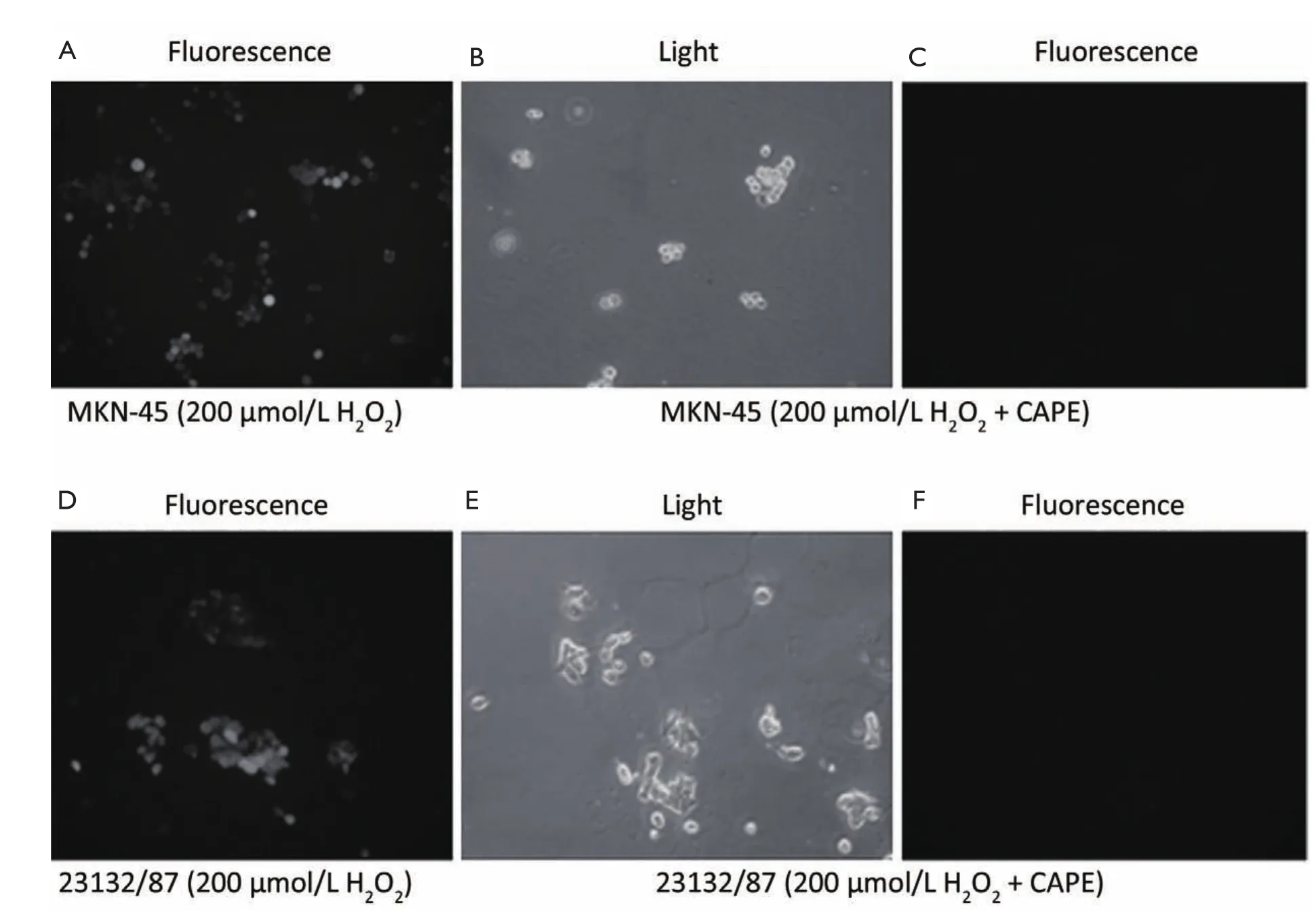
Figure 4 Analysis of internal oxidative stress in only 200 μmol/L H2O2treated MKN-45 and 23132/87 (A, D) and CAPE treated cells which were exposed to 200 μmol/L H2O2initially (B, C, E, F). B and E show the light microscopic views while A, C, D and F show the fluorescent microscopic views of MKN-45 and 23132/87 cells lines under indicated conditions (200×)
Removal of oxidative stress with CAPE exposure
In both MKN-45 and 23132/87 cells, 24 h exposure to CAPE resulted in the removal of oxidative stress as shown by DCFH-DA method (Figure 4). And 200 μmol/L H2O2exposure revealed an intracellular accumulation of oxidative stress in both of the cell lines (Figure 4A,D). However, the fluorescent microscopic view of the cells (Figure 4C,F) of which light microscopic views were demonstrated (Figure 4B,E) clearly reveals that CAPE treatment removed the accumulated oxidative stress in the viable MKN-45 and 23132/87 cells.
Effect of CAPE treatment on expressions of MMP genes compared to oxidative stress conditions
In MKN-45 cells, the expressions ofMMP-1,MMP-7,MMP-14,MMP-15,MMP-17and β-catenin genes that were up-regulated consequent to H2O2exposure were down-regulated following inclusion of CAPE compared to the expression levels of the H2O2exposed cells. Likewise, in 23132/87 cells, a decrease only in the expression ofMMP-15gene after CAPE application was observed since it was the onlyMMPin this cell line that was affected by H2O2exposure (Table 3). β-catenin expression was 1.0 fold down-regulated significantly (P<0.05) following CAPE treatment compared to H2O2treated MKN-45 cells, while it was 2.4 fold decreased significantly (P<0.05) in 23132/87 cells. Moreover, theMMP-1andMMP-7genes that were expressed in MKN-45 but not in 23132/87 cells showed a significant decrease 4.7 and 8.7 (P<0.05), respectively, in MKN-45 cells following CAPE treatment compared to the H2O2exposed cells (Figure 5). Also, 1.3 and 10.8 fold significant decrease (P<0.05) in the expression ofMMP-15gene in MKN-45 and 23132/87 cells, respectively, was determined (Figure 5). The expressions ofMMP-14andMMP-17genes that were only expressed in MKN-45 cells were decreased significantly by 11.20 and 0.38 (38%)fold (P<0.05), respectively, after the removal of oxidative stress. On the other hand,MMP-28gene of which the expressions was demonstrated in both of the cell lines but not affected by H2O2exposure, showed 1.20 and 0.27 (27%) fold significant decrease (P<0.05) in expression after CAPE treatment (Figure 5).
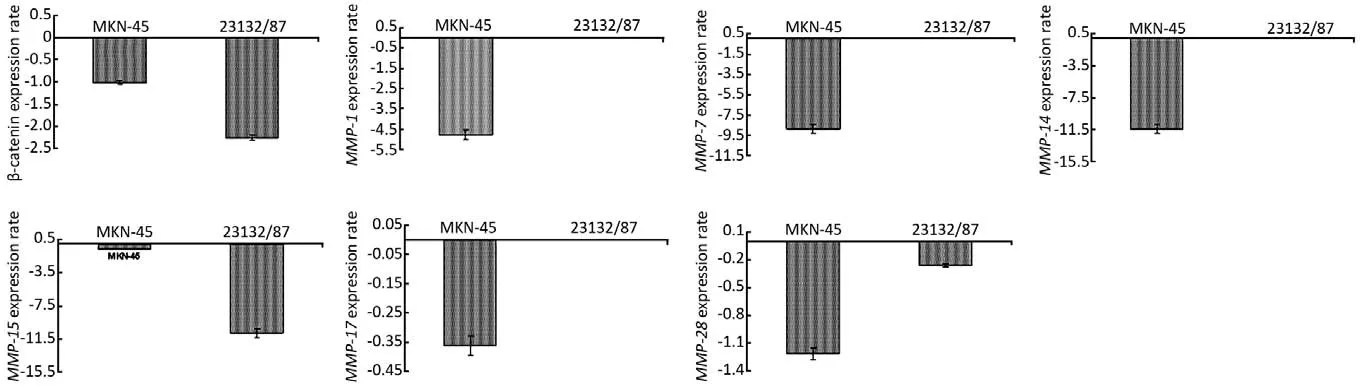
Figure 5 The expression rates of β-catenin,MMP-1,MMP-7,MMP-14,MMP-15,MMP-17andMMP-28genes in the cell lines treated with CAPE after 24 h of 200 μmol/L H2O2exposure compared with 200 μmol/L H2O2exposed control cells. Each of data is the average of three independent values and standard deviations are indicated above (σn-1) data values. All the down-regulations observed in the expressions were taken as statistically significant (P<0.05) as determined by studentt-test
Discussion
Extensive research to date suggests that continued oxidative stress can lead to chronic inflammation, which in turn could mediate most chronic diseases such as diabetes, cardiovascular, neurological, pulmonary diseases and cancers. Oxidative stress is linked to various steps involved in carcinogenesis, including cellular transformation, promotion, survival, proliferation, invasion, angiogenesis and metastasis (16,17).
As a tumor promoter, it leads to increased levels of oxidatively modified bases such as 8-OHdG, in the tissues which are exposed to oxidative stress (28-32). Gastric tissue is the primary site where continuous oxidative stress and chronic inflammation appear. In this study, likewise, we demonstrated the presence of 8-OHdG, an oxidative stress induced DNA base modification, in MKN-45 and 23132/87 cells lines suggesting the involvement of oxidative stress in gastric tumor promotion with abnormality in their morphologies and detachment from the surface. We also showed that H2O2exposure causes an accumulation of oxidative stress in the viable MKN-45 and 23132/87 cells and the removal of the oxidative stress with CAPE resulted in removal of the accumulated oxidative stress in these cells.
NF-κB, AP-1, P53, HIF-1α, PPAR-γ, β-catenin/Wnt, and Nrf2 are a variety of transcription factors which are activated by oxidative stress. The studies revealed that activation of these transcription factors can lead to the expression of over 500 different genes, including those for growth factors, cell cycle regulatory molecules, antiinflammatory molecules, inflammatory cytokines, and chemokines (1,2,10,11). Here, we analyzed the change in the expression pattern of β-catenin, an adhesion molecule and a transcription factor, after H2O2exposure of the gastric cancer cell lines, and we report that β-catenin expression increases in gastric cancer MKN-45 cells following H2O2exposure but not in 23132/87 cells.
Among the studiedMMPs,MMP-1,MMP-7,MMP-10,MMP-14andMMP-17genes were specifically expressed in MKN-45 cells. In these cells, parallel to β-catenin upregulation following H2O2treatment, a remarkable increase in the expressions of theseMMPgenes were also illustrated. Moreover, the removal of oxidative stress with CAPE caused a decrease in the expressions of the same genes. All these information suggestsMMP-1,MMP-7,MMP-14andMMP-17up-regulation is directly related with the exposure to oxidative stress. In addition to this, increase and decrease in β-catenin expression under oxidative stress and non-oxidative stress conditions, respectively, might be related to the increase in the expressions of theseMMPgenes. On the other hand,MMP-11,MMP-15,MMP-23andMMP-28were expressed in both of the cell lines. Among these, the expressions ofMMP-15was increased after exposure of MKN-45 and 23132/87 cells to H2O2while CAPE treatment reversed this effect and caused adecrease inMMP-15gene expression in both of the cell lines studied, which also reveals the link between oxidative stress andMMP-15gene expression. Interestingly, the down-regulation ofMMP-28expression in 23132/87 cells after CAPE treatment even though there was not an upregulation following H2O2exposure suggested an internal oxidative stress in this cell line prior to external H2O2exposure took role inMMP-28expression and removal of internal oxidative stress consequent to CAPE treatment resulted in the down-regulation ofMMP-28gene compared to the untreated control cells.
β-catenin-independent up-regulation of Wnt5A inducedMMP-1expression was observed in temporomandibular joint condylar chondrocytes (33). Here, we suggest that β-catenin might augment tumor invasion by increasing the rates of cell migration under oxidative stress conditions by promoting up-regulation ofMMP-1in gastric cancer MKN-45 cells. It is well established thatMMP-7is a Wntβ-catenin target (34,35) and our results about the parallel up-regulation of β-catenin andMMP-7following H2O2exposure of MKN-45 cells is expected. Up-regulation ofMMP-14can also be related to the up-regulation of β-catenin since there exist data that demonstrated the detection of Fhit and β-catenin at theMMP-14promoter in MCF-7 cells to regulate growth (36). On the other hand, there is no information about the regulation ofMMP-17by β-catenin, thus our results suggest a possible relation between the regulation ofMMP-17and β-catenin since under oxidative stress conditions, the expression of the genes increased, while the removal of oxidative stress caused the opposite.
In support of our data with change in the expressions ofMMPgenes,MMP-13,MMP-3andMMP-10were remarkably up-regulated by oxidant directly, and their activities were implicated in the invasive potential induced in NMuMG cells (37). Likewise,MMP-2andMMP-9were activated post-transcriptionally by prolonged oxidative treatment (38). The activation ofMMPs, such asMMP-2, probably occurs by the reaction of ROS with thiol groups in the protease catalytic domain (39). In addition to their role as key regulators ofMMPactivation, ROS have been implicated inMMPgene expression (40). Both H2O2and nitric oxide donors, as well as the increased expression of iNOS, stimulate the expression of severalMMPs(MMP-1,MMP-3,MMP-9,MMP-10andMMP-13) (40). In fibroblastic cells, the sustained production of H2O2recently was shown to activateMMP-2and to increase cell invasion (41). Oxidative stress may also modulateMMPexpression by activation of Ras, or direct activation of the MAPK family members ERK1/2, P38, and JNK, or inactivation of phosphatases that regulate these proteins (42).
In conclusion, our data related to the change in the expressions of β-catenin and severalMMPgenes following oxidative stress exposure to gastric cancer cell lines give an idea about the effect of oxidative stress on the expressions of these genes which will be a hot spot research in gastric carcinogenesis at the molecular level in near future and take the attention of the researchers in this area since gastric mucosa is continuously exposed to oxidative stress and these genes are the main modulators of invasion and metastasis.
Acknowledgements
This project was granted by the Scientific and Technical Research Council of Turkey (TUBITAK) under the project number 105S352 (SBAG-K-110) and by the Scientific Research Fund of Fatih University under the project number P50030703.
Disclosure:The authors declare no conflict of interest.
1. Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010;49:1603-16.
2. Cortes DF, Sha W, Hower V, et al. Differential gene expression in normal and transformed human mammary epithelial cells in response to oxidative stress. Free Radic Biol Med 2011;50:1565-74.
3. D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 2007;8:813-24.
4. Morel Y, Barouki R. Repression of gene expression by oxidative stress. Biochem J 1999;342:481-96.
5. Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol 2005;18:1779-91.
6. Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 2005;7:385-94.
7. Slupphaug G, Kavli B, Krokan HE. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res 2003;531:231-51.
8. Clopton DA, Saltman P. Low-level oxidative stress causes cell-cycle specific arrest in cultured cells. Biochem Biophys Res Commun 1995;210:189-96.
9. Chua PJ, Yip GW, Bay BH. Cell cycle arrest induced by hydrogen peroxide is associated with modulation of oxidative stress related genes in breast cancer cells. Exp Biol Med (Maywood) 2009;234:1086-94.
10. Seng S, Avraham HK, Jiang S, et al. The nuclear matrix protein, NRP/B, enhances Nrf2-mediated oxidative stress responses in breast cancer cells. Cancer Res 2007;67:8596-604.
11. Almeida M, Han L, Martin-Millan M, et al. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 2007;282:27298-305.
12. Hoogeboom D, Essers MA, Polderman PE, et al. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem 2008;283:9224-30.
13. Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol 2007;9:210-7.
14. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003;3:276-85.
15. Sanders LM, Henderson CE, Hong MY, et al. Pro-oxidant environment of the colon compared to the small intestine may contribute to greater cancer susceptibility. Cancer Lett 2004;208:155-61.
16. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7.
17. Mantovani A. Cancer: Inflammation by remote control. Nature 2005;435:752-3.
18. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011;278:16-27.
19. Farinati F, Cardin R, Cassaro M, et al. Helicobacter pylori, inflammation, oxidative damage and gastric cancer: a morphological, biological and molecular pathway. Eur J Cancer Prev 2008;17:195-200.
20. Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene 2000;19:1102-13.
21. McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet 2002;3:737-47.
22. Ito E, Yana I, Fujita C, et al. The role of MT2-MMP in cancer progression. Biochem Biophys Res Commun 2010;393:222-7.
23. Lowy AM, Clements WM, Bishop J, et al. beta-Catenin/ Wnt signaling regulates expression of the membrane type 3 matrix metalloproteinase in gastric cancer. Cancer Res 2006;66:4734-41.
24. Tang CH, Yamamoto A, Lin YT, et al. Involvement of matrix metalloproteinase-3 in CCL5/CCR5 pathway of chondrosarcomas metastasis. Biochem Pharmacol 2010;79:209-17.
25. Bass DA, Parce JW, Dechatelet LR, et al. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol 1983;130:1910-7.
26. Gencer S, Cebeci A , Irmak-Yazicioglu MB. Silencing of the MMP-3 gene by siRNA transfection in gastric cancer AGS cells. J Gastrointestin Liver Dis 2011;20:19-26.
27. Gencer S, Irmak Yazicio?lu MB. Differential response of gastric carcinoma MKN-45 and 23132/87 cells to H2O2exposure. Turk J Gastroenterol 2011;22:145-51.
28. Wei H, Frenkel K. Suppression of tumor promoterinduced oxidative events and DNA damage in vivo by sarcophytol A: A possible mechanism of anti-promotion. Cancer Res 1992;52:2298-303.
29. Hattori-Nakakuki Y, Nishigori C, Okamoto K, et al. Formation of 8-hydroxy-2'-deoxyguanosine in epidermis of hairless mice exposed to near-UV. Biochem Biophys Res Commun 1994;201:1132-9.
30. Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res 1994;305:253-64.
31. Rosin MP, Saad el Din Zaki S, Ward AJ, et al. Involvement of inflammatory reactions and elevated cell proliferation in the development of bladder cancer in schistosomiasis patients. Mutat Res 1994;305:283-92.
32. Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocyte–generated oxidants in carcinogenesis. Blood 1990;76:655-63.
33. Ge X, Ma X, Meng J, et al. Role of Wnt-5A in interleukin-1 beta-induced matrix metalloproteinase expression in rabbit temporomandibular joint condylar chondrocytes. Arthritis Rheum 2009;60:2714-22.
34. Crawford HC, Fingleton BM, Rudolph-Owen LA, et al. The metalloproteinase matrilysin is a target of betacatenin transactivation in intestinal tumors. Oncogene 1999;18:2883-91.
35. Brabletz T, Jung A, Dag S, et al. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 1999;155:1033-8.
36. Weiske J, Albring KF, Huber O. The tumor suppressor Fhit acts as a repressor of beta-catenin transcriptional activity. Proc Natl Acad Sci USA 2007;104:20344-9.
37. Mori K, Shibanuma M, Nose K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res 2004;64:7464-72.
38. Westermarck J, Kahari V. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 1999;13:781-92.
39. Rajagopalan S, Meng XP, Ramasamy S, et al. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 1996;98:2572-9.
40. Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med 2004;37:768-84.
41. Yoon SO, Park SJ, Yoon SY, et al. Sustained production of H(2)O(2) activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/ NF-kappa B pathway. J Biol Chem 2002;277:30271-82.
42. Storz P. Reactive oxygen species in tumor progression. Front Biosci 2005;10:1881-96.
Cite this article as:Gencer S, Cebeci A, Irmak-Yazicioglu MB. Matrix metalloproteinase gene expressions might be oxidative stress targets in gastric cancer cell lines. Chin J Cancer Res 2013;25(3):322-333. doi: 10.3978/j.issn.1000-9604.2013.06.05

10.3978/j.issn.1000-9604.2013.06.05
Submitted Mar 19, 2013. Accepted for publication May 24, 2013.
 Chinese Journal of Cancer Research2013年3期
Chinese Journal of Cancer Research2013年3期
- Chinese Journal of Cancer Research的其它文章
- Intrinsic apoptotic pathway and G2/M cell cycle arrest involved in tubeimoside I-induced EC109 cell death
- Effect of early enteral nutrition on postoperative nutritional status and immune function in elderly patients with esophageal cancer or cardiac cancer
- New frontiers in peritoneal malignancies
- Pharmacological blockage of CYP2E1 and alcohol-mediated liver cancer: is the time ready?
- Risk factors associated with early recurrence of adenocarcinoma of gastroesophageal junction after curative resection
- Hedgehog signaling pathway and ovarian cancer
