Short-term entecavir versus lamivudine therapy for HBeAg-negative patients with acute-onchronic hepatitis B liver failure
Guangzhou, China
Short-term entecavir versus lamivudine therapy for HBeAg-negative patients with acute-onchronic hepatitis B liver failure
Jing Lai, Ying Yan, Li Mai, Yu-Bao Zheng, Wei-Qiang Gan and Wei-Min Ke
Guangzhou, China
BACKGROUND:Selection of drugs for antiviral therapy of patients with hepatitis B virus (HBV)-related acute-onchronic liver failure (ACLF) remains diff i cult. This study was undertaken to evaluate the short-term eff i cacy of entecavir versus lamivudine on hepatitis B e antigen (HBeAg)-negative patients with ACLF.
METHODS:The data of 182 HBeAg-negative patients with ACLF were retrospectively collected from patient prof i les of the hospital. In these patients, 93 HBeAg-negative patients with ACLF were treated orally with 0.5 mg of entecavir and 89 were treated orally with 100 mg of lamivudine every day. The gender and age were matched between the two groups. Biochemical items, the model for end-stage liver disease (MELD) score, and HBV DNA level were matched at baseline between the two groups and monitored during treatment. The 3-month mortalities of the two groups were compared.
RESULTS:No signif i cant differences were found in biochemical items, MELD score, and HBV DNA level at baseline (P>0.05). HBV DNA level decreased within 3 months in both groups (P<0.05), regardless of the pretreatment MELD score. In patients with the same range of pretreatment MELD scores, treatment duration, posttreatment HBV DNA levels, percentage of HBV DNA level <2.7 lg copies/mL, biochemical items, MELD scores and 3-month mortality were similar in the two groups (allP>0.05). Pretreatment MELD score was not related to posttreatment HBV DNA levels (P>0.05), but related to a 3-month mortality in both groups (bothP<0.001).
CONCLUSIONS:In HBeAg-negative patients with ACLF, the short-term eff i cacy of entecavir versus lamivudine was similar. The degree of pretreatment liver failure signif i cantly affected the outcome of treatment.
(Hepatobiliary Pancreat Dis Int 2013;12:154-159)
acute-on-chronic liver failure;hepatitis B virus; antiviral therapy; clinical analysis
Introduction
Acute-on-chronic liver failure (ACLF) is a clinical syndrome where acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease.[1]Chronic hepatitis B virus (HBV) infection is a major cause of ACLF in Asia.[2]The negative rate of hepatitis B e antigen (HBeAg) was reported to be 71.74% in 2008 in China and is increasing.[3]Precore or basal core promoter mutations of HBV promote virus replication, which is a major pathogenesis initiating factor and may cause progression of ACLF in HBeAg-negative patients.[4,5]Both entecavir (Baraclude Bristol-Myers Squibb) and lamivudine (Epivir-HBV, GlaxoSmithKline) are nucleoside analogues with potent and highly selective inhibition of HBV DNA polymerase,[6]but the short-term eff i cacy of entecavir versus lamivudine on this disease and the possible inf l uential factors remain elusive. The model for end-stage liver disease (MELD) scoring has been found to be related to prediction of residual liver function and of short-term (3-month) mortality among patients with end-stage liver disease.[7,8]
In this study, we used MELD scores to assess the degree of liver failure and to evaluate the short-term eff i cacy of entecavir versus lamivudine on treatmentnaive HBeAg-negative patients with ACLF. To collectmore convincing evidences, a well-designed matched retrospective study method was used to control the bias of patient selection.
Methods
Patients
Clinical data of 182 HBeAg-negative patients with ACLF treated between January 2003 and December 2011 at our department were collected. Inclusion criteria were as follows: (i) acute hepatic insult manifesting as jaundice (serum bilirubin ≥5 mg/dL) and coagulopathy [international normalized ratio (INR) ≥1.5 or prothrombin activity <40%], complicated within 4 weeks by ascites and/or encephalopathy;[1](ii) the presence of hepatitis B surface antigen (HBsAg) in the serum for at least 6 months; (iii) HBeAg being negative before treatment; and (iv) evidence of active viral replication as documented by measurable HBV DNA in the serum (≥2.7 lg copies/mL). Exclusion criteria were: (i) super-infection or co-infection with hepatitis A, C, D, E viruses, Epstein-Barr virus (EBV), cytomegalovirus or human immunodef i ciency virus (HIV); (ii) a previous course of any antiviral, immunomodulator or cytotoxic/ immunosuppressive therapy for chronic hepatitis within at least the preceding 12 months; (iii) evidence of decompensated liver disease before enrollment; (iv) hepatocellular carcinoma diagnosed by ultrasonography or computed tomography; (v) coexistence of any other serious medical diseases and other liver diseases such as autoimmune hepatitis, alcoholic liver disease, drug hepatitis or Wilson's disease; and (vi) the malignant jaundice induced by obstructive jaundice or hemolytic jaundice and prolonged prothrombin time induced by blood system disease.
Of the 182 patients, 93 patients were treated orally with 0.5 mg of entecavir and 89 patients were treated orally with 100 mg of lamivudine every day. The two groups were matched for gender, age, serum HBV DNA level, biochemical items [total bilirubin (TBiL), creatine, and INR] and MELD score at baseline. If a patient died within 3 months, the date of death was regarded as the primary endpoint to calculate the duration of therapy. Post-treatment HBV DNA level, biochemical values and MELD score were collected before death. If the patient survived over 3 months, HBV DNA level and biochemical items were detected after a 3-month antiviral therapy.
The study was conducted in accordance with the ethics principles of theDeclaration of Helsinki, the Good Clinical Practice guidelines and applicable local regulatory requirements. Written informed consent was obtained from all patients or their relatives before treatment.
Laboratory measurements
The following equation was used: MELD score=3.8× Ln (TBiL [mg/dL]) + 11.2 ×Ln (INR) + 9.6 ×Ln (creatinine [mg/dL]) + 6.4 × etiology score (0 if cholestatic or alcoholic, 1 otherwise).[9]
Serum HBeAg was detected by an AxSYM automatic rapid immunoassay system (Abbott Diagnostics). Serum HBV DNA was measured by PCR assay (Amplicor HBV Monitor Test; Daan gene Diagnostics, Guangzhou, China). The detection limit was ≥2.7 lg copies/mL.
Tyrosine, methionine, aspartate and aspartate (YMDD) motif mutant was detected by PCR-f l uorescence for mixed viral-genotype populations (wild-type and mutant virus) at baseline and follow-up. The detection limit was≥3.00 lg copies/mL.
Mortality analysis
All the patients were followed up and the outcome of treatment was recorded. The date and cause were documented for each death.
Statistical analysis
The data of the patients were presented as mean ± standard deviation (SD). Statistical analyses were made using SPSS version 18.0 (SPSS Inc., Chicago, Illinois, USA). The rates were compared using the Chi-square test. The measurement results were compared by independentttest between the two groups and by oneway ANOVA among three ranges. APvalue less than 0.05 was considered statistically signif i cant.
Results
Demographic characteristics of the patients at baseline
The baseline characteristics of the patients are summarized in Table 1. The pretreatment HBV DNA level of each patient was above 2.7 lg copies/mL. Regardless of the pretreatment MELD score, no signif i cant difference was found in gender, age, serum HBV DNA level, biochemical items and MELD score between the two groups (P>0.05).
Posttreatment characteristics of the patients
No signif i cant differences were found in treatment duration, HBV DNA level, HBV DNA level <2.7 lg copies/mL, biochemical characteristics (TBiL, creatine,and INR) and MELD score between the two groups regardless of pretreatment MELD scores (allP>0.05) (Table 2). The levels of HBV DNA signif i cantly decreased after treatment in the two groups (bothP<0.05), but this was not signif i cantly related to pretreatment MELD score (allP>0.05) (Tables 3 and 4). In patients with pretreatment MELD scores of 23-30 or above 30, MELD score, TBiL level and INR were not changed signif i cantly after treatment in both groups (P>0.05); whereas in patients with pretreatment MELD scores below 23, MELD score and TBiL level decreased signif i cantly after treatment in both groups (bothP<0.05) (Tables 3 and 4).
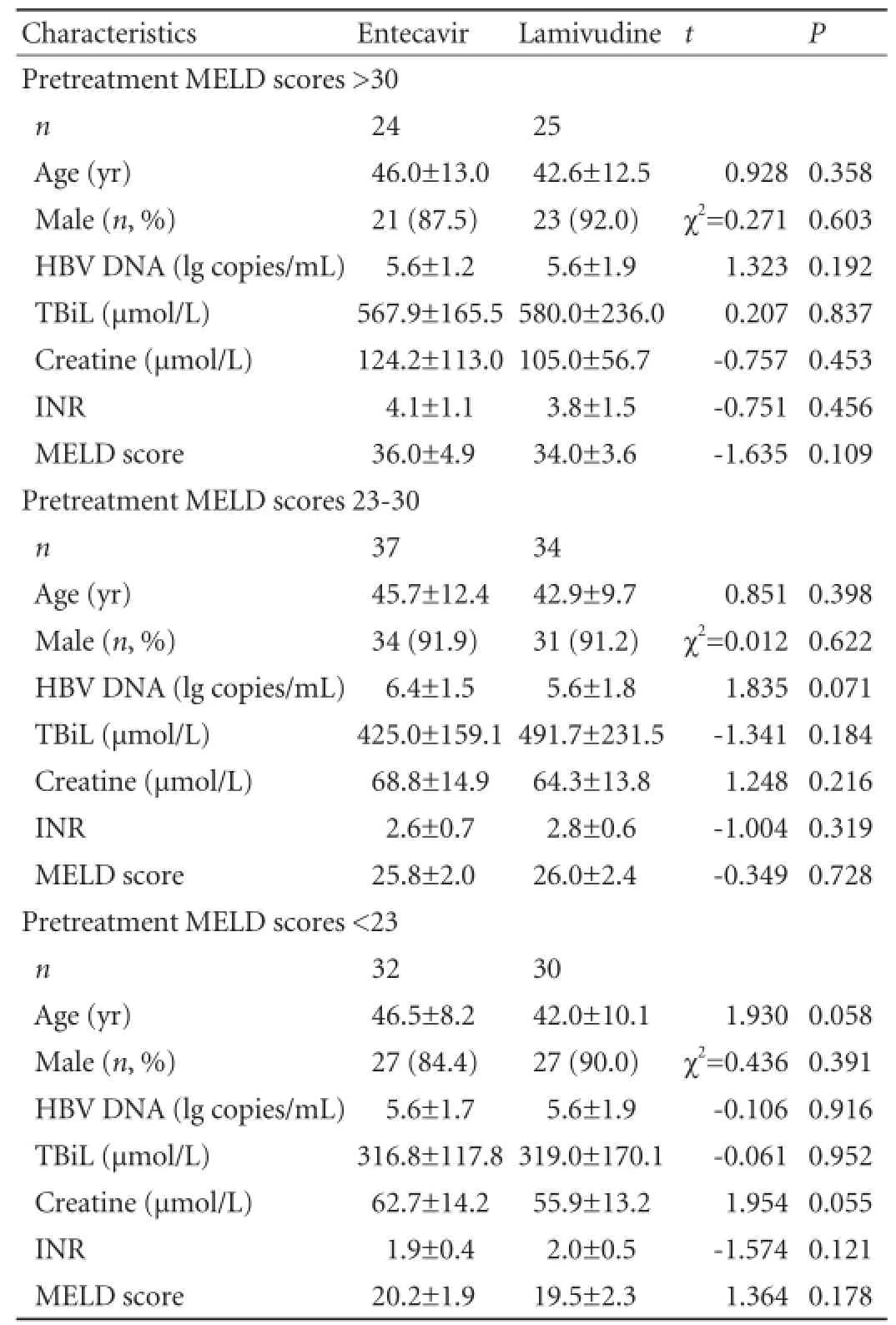
Table 1.Demographic and baseline characteristics of the patients
Mortality
During follow-up, 89 patients died from liver failure. For patients with the same range of pretreatment MELD score, the 3-month mortality was similar in the twogroups (P>0.05). However, the lower pretreatment MELD score was related to a lower mortality in both groups (P<0.05) (Tables 3 and 4).
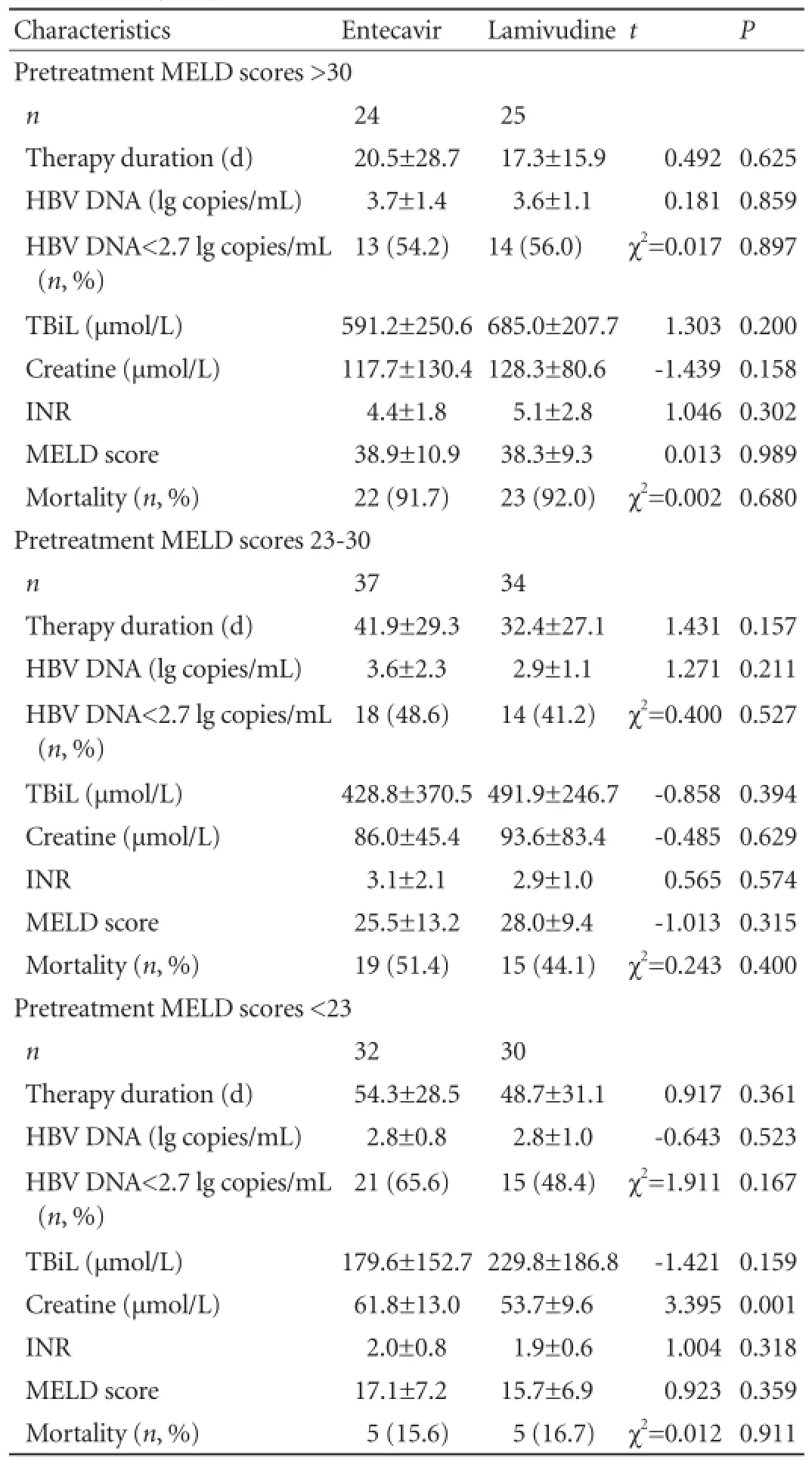
Table 2.Comparisons of virological, biochemical characteristics and mortality of patients after treatment
YMDD mutations and safety
None of the patients showed evidence of YMDD mutations at baseline or during treatment. No serious adverse event was attributed to lamivudine or entecavir. All the patients were tolerated to the treatment without modif i cation of dose or early discontinuation.

Table 3.Pre- and posttreatment virological and biochemical characteristics and mortality in entecavir group
Discussion
Long-term follow-up studies have demonstrated a close relationship between disease severity and viral factors in chronic HBV infection.[10,11]HBeAg-negative patients with chronic hepatitis B differ from HBeAgpositive patients, whose liver disease tends to fl uctuate and is more advanced.[11-13]Lai et al[14]reported that entecavir resulted in signif i cantly higher rates of histologic, virologic and biochemical improvement than did lamivudine after 48 weeks of treatment in patients with HBeAg-negative chronic hepatitis B who had not previously received a nucleoside analogue. Some studies[2,3,15,16]found that both entecavir and lamivudine were effective in treating HBeAg-negative patients with ACLF. Patients treated with either of the two agentsshowed a signif i cant decrease of HBV DNA level and lower clinical and biochemical responses within 4 weeks as compared with those who were not treated with nucleoside analogue. The latter could be evaluated by changes in MELD score.[2,15,16]However, the difference between the two agents in treating HBeAg-negative patients with ACLF remains unclear.
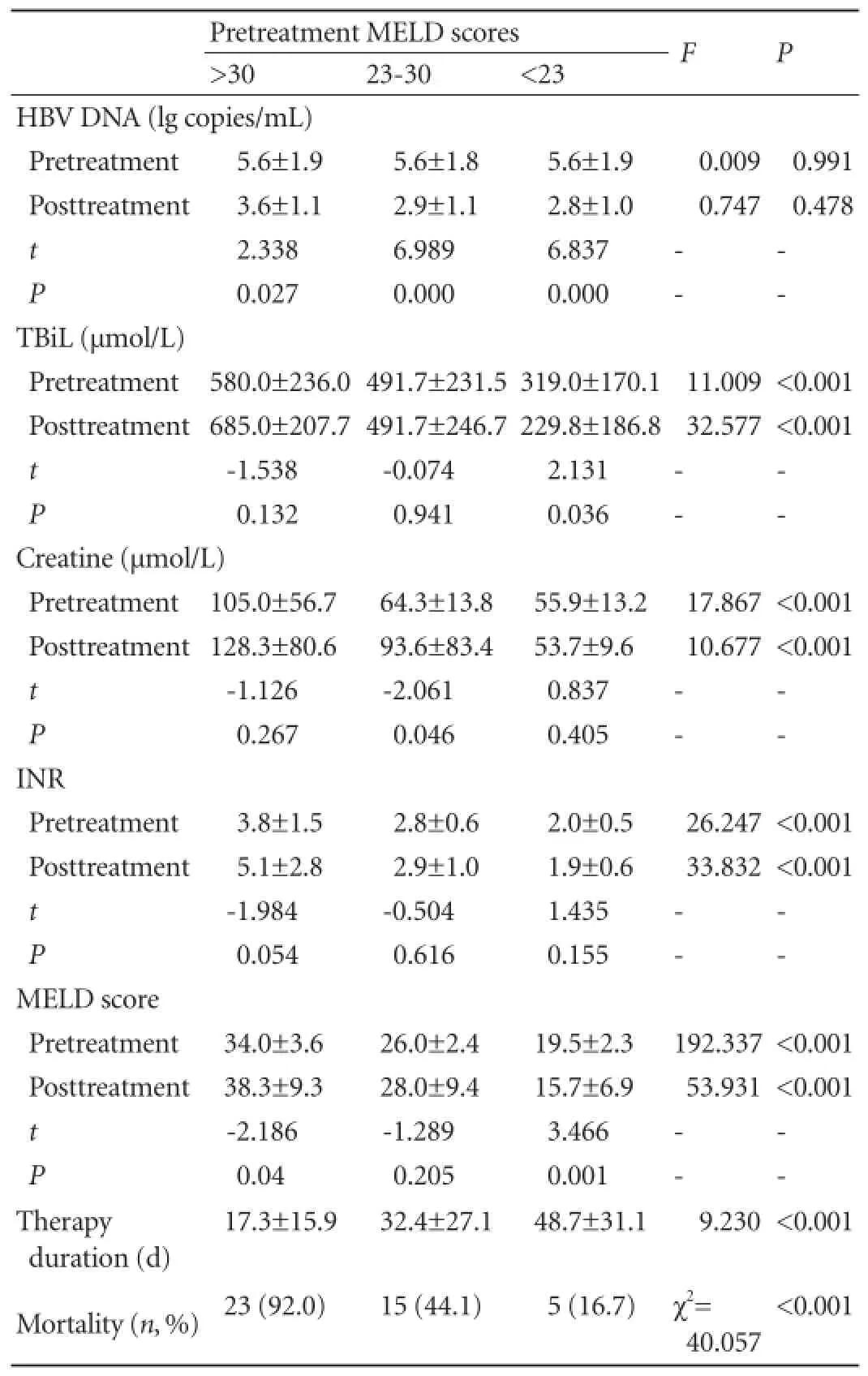
Table 4.Pre- and posttreatment virological and biochemical characteristics and mortality in the lamivudne group
In the present study, we used a well-designed matched method to control the bias of patient selection. Either entacavir or lamivudine rapidly and signif i cantly decreased the levels of HBV DNA. However, posttreatment HBV DNA levels showed no signif i cant relationship with pretreatment MELD scores (P>0.05). In patients with the same range of pretreatment MELD scores, no signif i cant differences were observed in posttreatmentHBV DNA levels and percentage of HBV DNA level <2.7 lg copies/mL between the two groups (P>0.05). If the duration of treatment was the same, similar effect of the two agents was seen in lowering HBV DNA level regardless of the degree of liver failure.
In patients with the same range of pretreatment MELD scores, posttreatment biochemical items, MELD scores and mortality were similar in the two groups (P>0.05). Posttreatment biochemical values and MELD scores increased following the decline of HBV DNA level in patients with pretreatment MELD scores below 23. Patients with a MELD score below 23 were at an early stage of ACLF. Over 60% of them could survive longer with various supporting and systematic therapies including antiviral therapy. After that the HBV DNA levels decreased, immune compromise gradually improved and liver regeneration appeared. The patients recovered after improvement of liver function. The patients with MELD scores of 23-30 were at a middle stage of ACLF. A higher MELD score ref l ected more immune compromise and more severe hepatic necrosis. The prognosis of the disease depended on individual disparity in immune response and liver regeneration. The patients with a MELD score over 30 were at a late stage of ACLF, and factors other than viral replication had a great impact on the prognosis. The livers of these patients had already undergone massive or submassive hepatic necrosis. Suppressing viral replication at this late stage was unlikely to be effective, as the decline of HBV DNA level was due to immune clearance, hepatic necrosis and drug eff i cacy. The main determinants for recovery were liver regeneration and the rapid cessation of ongoing necrotizing inf l ammation. Patients often died from severe complications rapidly before antiviral therapy could have any effect. This is why some patients' condition deteriorated even though the replication of HBV had been suppressed. Our study also found a signif i cant relationship between pretreatment MELD scores and mortality, which was consistent with previous studies.[17,18]MELD score is related to the prognosis of the patients with ACLF. This was similar to the result reported elsewhere.[2]For those patients with a MELD score of above 30, the MELD score was the only independent predictor for the outcome of treatment.
Our study conf i rmed the role of HBV DNA in the pathogenesis of HBeAg-negative ACLF and the "two-hit" hypothesis. The fi rst hit is considered to be a primary injury caused directly or indirectly by increased HBV replication, and the second one is a cytokine-cored lesion.[19-21]HBV DNA may be a key factor for HBeAgnegative ACLF but the cytokine-cored secondary lesion is probably more important in the progression.
The main limitation of our study is its retrospective nature. However, patient material was large and we tried to match the groups in a controlled fashion.
In this study, the short-term eff i cacy of entecavir versus lamivudine was similar in previously untreated HBeAg-negative patients with ACLF. The severity of pretreatment liver failure signif i cantly affected the short-term outcome of both therapies. Therefore, early medical care is essential for all patients. Randomized trials are needed to evaluate the long-term eff i cacy, medical expense and drug resistance.
Contributors:KWM proposed the study. LJ and KWM performed research, collected data and wrote the fi rst draft. YY, ML, ZYB and GWQ collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. KWM is the guarantor.
Funding:None.
Ethical approval:The study was conducted in accordance with the ethics principles of theDeclaration of Helsinki, the Good Clinical Practice guidelines and applicable local regulatory requirements. Written informed consent was obtained from all patients or their relatives before treatment.
Competing interest:No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacif i c Association for the study of the liver (APASL). Hepatol Int 2009;3:269-282.
2 Sun LJ, Yu JW, Zhao YH, Kang P, Li SC. Inf l uential factors of prognosis in lamivudine treatment for patients with acuteon-chronic hepatitis B liver failure. J Gastroenterol Hepatol 2010;25:583-590.
3 Shu X, Xu QH, Chen N, Zhang K, Li G. The clinic features and the short-term eff i cacy of entecavir treatment of the HBeAG negative acute-on-chronic hepatitis B liver failure. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2008; 22:481-483.
4 Tsai WL, Lo GH, Hsu PI, Lai KH, Lin CK, Chan HH, et al. Role of genotype and precore/basal core promoter mutations of hepatitis B virus in patients with chronic hepatitis B with acute exacerbation. Scand J Gastroenterol 2008;43:196-201.
5 Ren X, Xu Z, Liu Y, Li X, Bai S, Ding N, et al. Hepatitis B virus genotype and basal core promoter/precore mutations are associated with hepatitis B-related acute-on-chronic liver failure without pre-existing liver cirrhosis. J Viral Hepat 2010;17:887-895.
6 Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology 2007;45:1056-1075.
7 Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464-470.
8 Botta F, Giannini E, Romagnoli P, Fasoli A, Malfatti F, Chiarbonello B, et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut 2003;52:134-139.
9 Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864-871.
10 Liaw YF. Hepatitis B virus replication and liver disease progression: the impact of antiviral therapy. Antivir Ther 2006;11:669-679.
11 Fattovich G. Natural history of hepatitis B. J Hepatol 2003;39: S50-58.
12 Papatheodoridis GV, Hadziyannis SJ. Review article: current management of chronic hepatitis B. Aliment Pharmacol Ther 2004;19:25-37.
13 Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, et al. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol 2002;36:263-270.
14 Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011-1020.
15 Chen J, Han JH, Liu C, Yu RH, Li FZ, Li QF, et al. Short-term entecavir therapy of chronic severe hepatitis B. Hepatobiliary Pancreat Dis Int 2009;8:261-266.
16 Shu X, Xu QH, Chen N, Zhang K, Li G. The eff i cacy of entecavir treatment on acute-on-chronic liver failure in patients with hepatitis. Zhonghua Chuan Ran Bing Za Zhi 2009;27: 281-286.
17 Yu JW, Wang GQ, Li SC. Prediction of the prognosis in patients with acute-on-chronic hepatitis using the MELD scoring system. J Gastroenterol Hepatol 2006;21:1519-1524.
18 Yu JW, Wang GQ, Zhao YH, Sun LJ, Wang SQ, Li SC. The MELD scoring system for predicting prognosis in patients with severe hepatitis after plasma exchange treatment. Hepatobiliary Pancreat Dis Int 2007;6:492-496.
19 Wasmuth HE, Kunz D, Yagmur E, Timmer-Strangh?ner A, Vidacek D, Siewert E, et al. Patients with acute on chronic liver failure display "sepsis-like" immune paralysis. J Hepatol 2005;42:195-201.
20 Ambrosino G, Naso A, Feltracco P, Carraro P, Basso SM, Varotto S, et al. Cytokines and liver failure: modif i cation of TNF- and IL-6 in patients with acute on chronic liver decompensation treated with Molecular Adsorbent Recycling System (MARS). Acta Biomed 2003;74:7-9.
21 Zhu CL, Yan WM, Zhu F, Zhu YF, Xi D, Tian DY, et al. Fibrinogen-like protein 2 fi broleukin expression and its correlation with disease progression in murine hepatitis virus type 3-induced fulminant hepatitis and in patients with severe viral hepatitis B. World J Gastroenterol 2005;11:6936-6940.
Received August 2, 2012
Accepted after revision January 4, 2013
AuthorAff i liations:Department of Infectious Diseases, Third Aff i liated Hospital, Sun Yat-Sen University, Guangzhou 510630, China (Lai J, Yan Y, Mai L, Zheng YB, Gan WQ and Ke WM)
Wei-Min Ke, MD, Department of Infectious Diseases, Third Aff i liated Hospital, Sun Yat-Sen University, Guangzhou 510630, China (Tel: 86-20-85253171; Fax: 86-20-85252559; Email: kwm1999@ 163.com)
? 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60025-9
 Hepatobiliary & Pancreatic Diseases International2013年2期
Hepatobiliary & Pancreatic Diseases International2013年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Ethyl pyruvate prevents in flammatory factors release and decreases intestinal permeability in rats with D-galactosamine-induced acute liver failure
- Technical note on complete excision of choledochal cysts
- Risk factors of choledocholithiasis formation after liver transplantation
- Hepaticojejunostomy with the "Hand-Fan" technique
- Values of circulating GPC-3 mRNA and alpha-fetoprotein in detecting patients with hepatocellular carcinoma
- Management hepatolithiasis with operative choledochoscopic FREDDY laser lithotripsy combined with or without hepatectomy
