Salting-out Extraction of 2,3-Butanediol from Jerusalem artichoke-based Fermentation Broth*
DAI Jianying (戴建英), ZHANG Yuanli (張媛麗) and XIU Zhilong (修志龍)**
School of Life Science and Biotechnology, Dalian University of Technology, Dalian 116024, China
1 INTRODUCTION
The utilization of lignocellulosic materials in biorefinery introduces more components into the fermentation broth than starch-based fermentation, thus making the recovery of bio-based chemicals from the fermentation broths more difficult. 2,3-Butanediol(2,3-BD) is one of the typical bio-based chemicals that can be produced from the fermentation of natural materials such as corncob [1] and Jerusalem artichoke tuber and stalk [2, 3]. Traditionally, the isolation of 2,3-BD from the fermentation broth begins with the removal of solid matters in the broth by high-speed centrifugation or membrane filtration, followed by methods such as solvent extraction, salting-out and pervaporation [4-7]. However, if the fermentation broth is prepared from natural substrates, it would be very viscous and the use of membrane filtration to remove the solid matters from the broth could quickly block the membrane [5]. Solvent extraction requires a very large amount of solvent, and the yield of 2,3-BD obtained is only about 75% since 2,3-BD is a hydrophilic molecule [7]. Alternatively, the use of salting-out has resulted in a 2,3-BD recovery of 94%-96%, but the operation of this method is rather tedious, and is only suitable in the laboratory [8]. Combining solvent extraction and salting-out, we have developed a novel method-salting-out extraction (SOE) (formerly called aqueous two-phase extraction) to separate 2,3-BD from glucose-based fermentation broth [9, 10]. During SOE, 2,3-BD is concentrated in the top phase, whereas cells and most proteins are removed from the top phase due to the precipitation of organic solvent and salting-out of inorganic salt, and most of the residual sugars are partitioned to the bottom phase. Thus, solid-liquid separation, removal of impurities and the concentrating of 2,3-BD can be achieved in one-step operation.
In this paper, viscous fermentation broth prepared from Jerusalem artichoke tuber and stalk by SSF (simultaneous saccharification and fermentation), which contained unhydrolyzed raw materials and high density of cells was subjected to SOE aiming to isolate 2,3-BD from the viscous broth. About 98% of solid matters was removed and 99% of 2,3-BD was recovered. This study presented a simple method for the separation of 2,3-BD directly from lignocellulosederived fermentation broth.
2 EXPERIMENTAL SECTION
2.1 Materials and Methods
Klebsiella pneumoniaeCICC 10011 was obtained from China Center of Industrial Culture Collection (Beijing, China). Cellulase and xylanase were purchased from Wuxi Xuemei Enzyme Co. (Wuxi,China), and inulinase was donated by Dalian Institute of Chemical Physics, Chinese Academy of Sciences(Dalian, China). 2,3-BD standard was purchased from Hongtai Biological Co., Ltd, China. Acetoin was purchased from Xiangyuan Chemical Industrial Co., Ltd,China. The cellulose triacetate hollow fiber dialyzer with effective surface area of 1.5 m2and cut-off relative molecular mass of 5000 Da was manufactured by NISSHO Corp., Osaka, Japan. All the other chemicals were of analytical grade.
2.2 Experimental procedure
2.2.1Preparation of fermentation broths
The fermentation broth used in this study was prepared from Jerusalem artichoke stalk and tuber.Jerusalem artichoke stalk consists of 25.6% hemicellulose, 43.7% cellulose and 14.1% lignin, as based on dry solid. The stalks were pretreated with 1% sulfuric acid, followed by addition of cellulase and xylanase to perform the enzymatic hydrolysis as described by Liet al. [2]. The mixture was used directly in fed-batch SSF withKlebsiella pneumoniaeCICC 10011 as the fermenting bacterial strain. Jerusalem artichoke tubers and enzymes (xylanase, cellulase and inulinase) were periodically fed to the bioreactor during the fermentation process. Fermentation was terminated after 72 h,and the fermentation broth was processed as described below.
2.2.2Salting-out extraction (SOE) of 2,3-butanediol and scale up
SOE was first carried out with pretreated fermentation broth (centrifuged at 6800×gfor 30 min followed by membrane filtration) at a 10-g scale, using 14%-23% K2HPO4/8%-26% ethanol (mass fraction). Appropriate amounts of pretreated fermentation broth and solid salt were mixed in a 10-ml graduated tube. The mixture was vortexed for 1 min, and ethanol was then added into the tube to a total mass of 10 g consisting of fermentation broth, salt and ethanol, followed by additionally vortexing for 2 min, and finally allowed to stand for 8-10 h at 25 °C. The concentrations of target products (2,3-BD and acetoin) in the top and bottom phases were analyzed by GC. The operation procedure of SOE used for isolating 2,3-BD from the untreated fermentation broth was the same as described above. However, due to the presence of large amount of solid materials, the phase separation was not clearly observed. After standing for 8-10 h,low-speed centrifugation (2260×gfor 20 min) was carried out to facilitate phase separation. Two concentrations of salt, each with various ethanol concentrations were explored at a 10-g scale, and the optimal systems were selected and applied to a 30-g and 400-g scale. The partition coefficient (K) is the ratio of concentration of product in top phase to that in bottom phase. The yield (Y) is the mass ratio of product in top phase to that in the combined top and bottom phases.The concentration multiple (N) is the concentration ratio of target product in top phase to that in the original fermentation broth. The selective coefficient of 2,3-BD to reducing sugars (Ks) was defined as the ratio of the partition coefficient of 2,3-BD to that of reducing sugars. The removal ratio (R) of soluble proteins in the top phase was defined as follows:R=(1-M/Mt)×100%, whereMandMtare the amount of protein in the top phase and the amount of total protein in the system, respectively. All SOE experiments were performed three times.
2.3 Analytical methods
2,3-BD, acetoin and ethanol were determined by gas chromatography (GC) (SHIMADZU GC-2010,Japan) with FID detector according to the published method [2]. The concentration of protein was measured by Bradford method with BSA (Bovine serum albumin) as a standard. The concentration of solid impurities was determined by measuring the optical density at 650 nm (OD650). The dry mass (DM) of solid impurities was calculated from a calibration equation [Y=3.5864X-9.9543,Yis the concentration of solid impurities,Xis the value of OD650(7<X<56)]which was obtained from the experimental curve of OD650vs. DM. To maintain a similar degree of precipitation of solid matters among the different samples,all samples were diluted 40-fold with water and allowed to stand for 8 minutes prior to the measurement of their OD650. Reducing sugars were quantified by the 3,5-dinitrosalicylic acid method. Viscosity was determined by NDJ-I rotary viscometer at 25 °C. The contents of hemicellulose, cellulose and lignin fractions were determined according to the methods presented by van Soestet al[11, 12].
3 RESULTS AND DISCUSSION
3.1 Salting-out extraction (SOE) of 2,3-BD from fermentation broth
In previous SOE studies with glucose-based fermentation broth, the yields of 2,3-BD from the three most efficient systems (K2HPO4/ethanol, (NH4)2SO4/ethanol, and (NH4)2SO4/isopropanol) were all greater than 90% [9, 10, 13] and the highest yield, 98.1%, was obtained with K2HPO4/ethanol system. (NH4)2SO4is relatively cheap, while K2HPO4/ethanol system demonstrates the highest yield of 2,3-BD. Thus, these two systems were chosen for the separation of 2,3-BD from Jeruslem artichoke-based fermentation broth.The compositions of the broths at the end of fermentation are listed in Table 1. The content of solid residues(cells and unhydrolyzed material measured as dry mass)in the broth was 187.7 g·L-1(DM) and the compositions of hemicellulose, cellulose and lignin within this dry mass were 7.0%, 28.8% and 8.7%, respectively.
For the convenience of operation, the partition behavior of 2,3-BD was primarily explored with pretreated broth. The yield (Y) and partition coefficient (K)of 2,3-BD in (NH4)2SO4/ethanol system were below 90% and 5, respectively, while in the K2HPO4/ethanol system they exceeded 95% and 20, respectively. Thus,the K2HPO4/ethanol system was selected for subsequent experiments. Such a difference in yield has also been observed in the separation of 1,3-propanediol from glycerol-based fermentation broth, and is related to the interaction between anions and H3O+[14]. The phosphate anion could form more hydrogen bonds with H3O+than sulphate anion, thus push more 2,3-BD to the top phase, and finally the recovery of 2,3-BD using K2HPO4/ethanol system was higher than that with (NH4)2SO4/ethanol system.
The results for separation of 2,3-BD and acetoin by the K2HPO4/ethanol system are shown in Fig. 1.
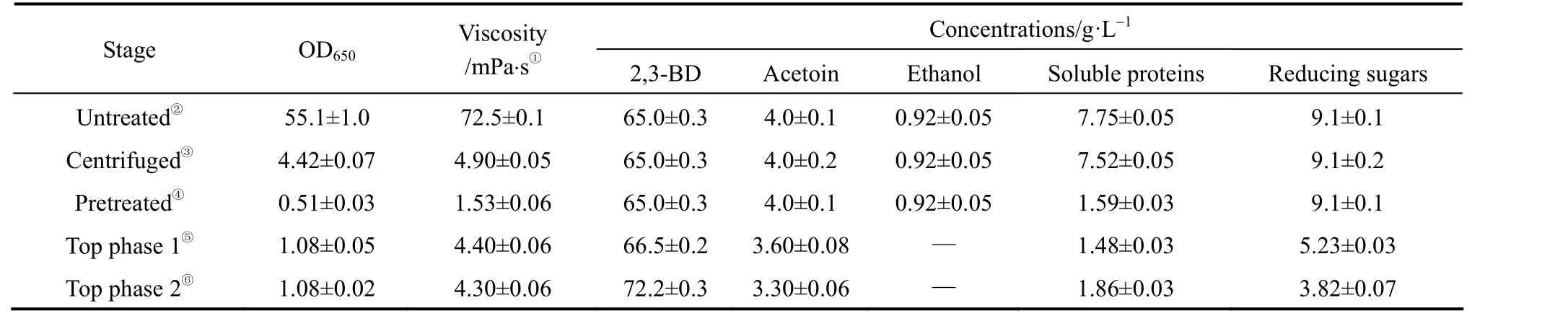
Table 1 Parameters of Jerusalem artichoke-based fermentation broth and the top phases after salting-out extraction
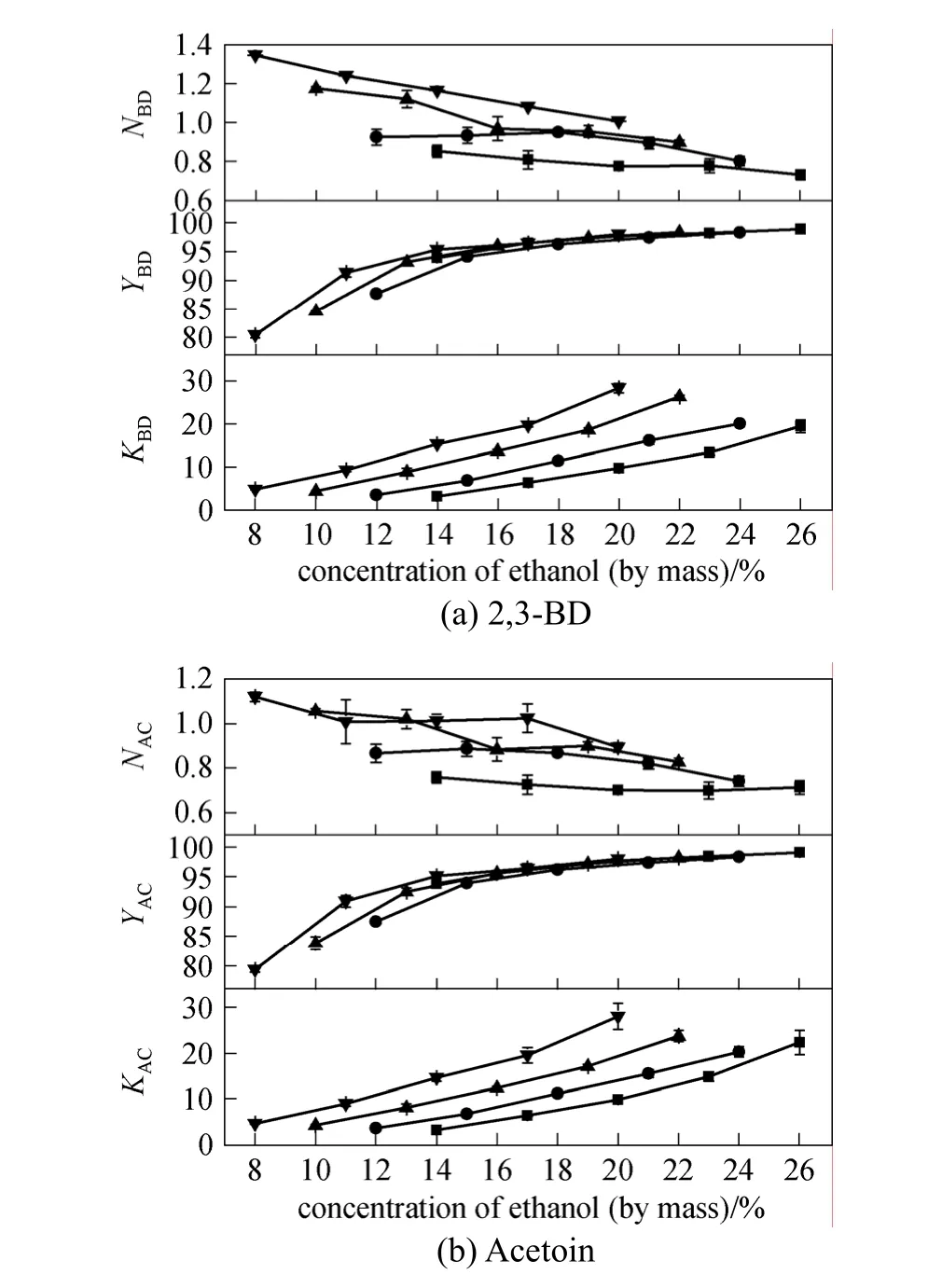
Figure 1 Effects of ethanol concentration on partition coefficient (K), yield (Y) and concentration multiple (N) for pretreated fermentation brothConcentrations of K2HPO4 (mass fraction)/%: ■ 14; ● 17;▲ 20; ▼ 23
By employing a combination of solvent extraction and salting-out, 2,3-BD and acetoin in the pretreated broth tended to distribute in the top phase consisting of ethanol and water. The concentrations of ethanol and salt greatly affected the partition of 2,3-BD and acetoin. The values ofYandKfor 2,3-BD and acetoin increased with increasing concentration of ethanol or salt, which were in good agreement with previous studies using glucose-based fermentation broths [9, 10, 13].The values ofYandKexceeded 95% and 20, respectively, when the ethanol concentration was 26%, 24%,22% and 20% for the K2HPO4concentration of 14%,17%, 20% and 23%, respectively. The maximum value ofYfor 2,3-BD was 98.96% obtained with 14%K2HPO4/26% ethanol (mass fraction), whereas the maximum value ofKwas 28.3, obtained with 23%K2HPO4/20% ethanol (mass fraction). The concentration multiple (N) for 2,3-BD and acetoin increased with increasing concentration of K2HPO4, and decreased as the concentration of ethanol increased. The maximum concentration multiple (N) for 2,3-BD was 1.35 under 23% K2HPO4/8% ethanol (mass fraction).The top phase could be distillated to recover the ethanol.During the process of salt extraction with azeotropic distillation, low concentration of salt is favorable for the recovery of ethanol [15]. The recovery of K2HPO4in the bottom phase would be conducted by acidification and ethanol dilution crystallization. About 78%K2HPO4in the bottom phase was recovered, which was much lower than that from the bottom phase of 1,3-propanediol (94.7% by methanol) [16]. The result was mainly caused by the differences in viscosity of the solution and the alcohol used. Considering the values ofYandN, K2HPO4concentrations of 20% and 17% (mass fraction) were chosen for the separation of 2,3-BD from untreated fermentation broth.
The SOE systems consisted of 20% K2HPO4/13%-22% ethanol (mass fraction) and 17% K2HPO4/15%-24% ethanol (mass fraction) were applied to the untreated fermentation broth, and the results are shown in Fig. 2. The values ofKandYas well asNfor 2,3-BD demonstrated similar behaviors as that for pretreated fermentation broth. The yield increased slowly when the concentration of ethanol reached a certain point. When the concentration of K2HPO4was 20% (mass fraction), the yield of 2,3-BD reached the highest (over 97%), and changed little when the ethanol concentration varied from 19% to 22% (mas fraction). The yield obtained with 17% (mass fraction)K2HPO4was almost the same. Considering the highNandYvalues for 2,3-BD, the concentrations of ethanol versus salt chosen as optimized conditions for the separation of 2,3-BD from the fermentation broth were 20%/19% (mass fraction) and 17%/21% (mass fraction) .
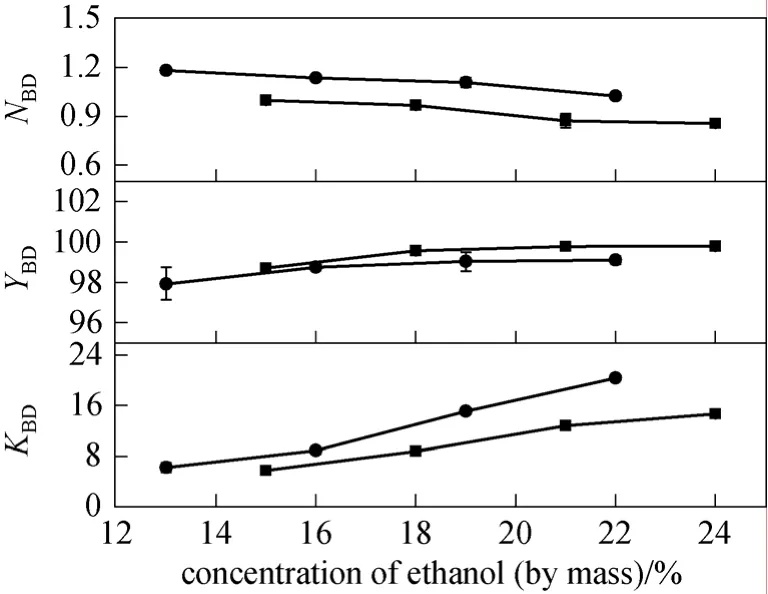
Figure 2 Effects of ethanol concentration on partition coefficient (KBD), yield (YBD) and concentration multiple(NBD) of 2,3-BD for untreated fermentation brothConcentrations of K2HPO4 (mass fraction)/%: ■ 17; ● 20
SOEs were carried out under optimized conditions with untreated fermentation broth at 10-g, 30-g and 400-g scales. The yields of 2,3-BD and acetoin were higher than 97% under both sets of conditions and remained almost constant as the system was scaled up(Table 2). At the 400-g scale, the yield of 2,3-BD obtained with both systems (97.7%versus99%) were similar to the yield obtained from glucose-based fermentation broth (99.6%) [9].
The partition coefficients of 2,3-BD and acetoin were a little lower than those obtained from pretreated broth (Fig. 1) because the untreated fermentation broth contained lots of solid residues, which affected the distribution of products in the top and bottom phases.When the composition of SOE system was 20%K2HPO4/19% ethanol (mass fraction), the partition coefficients for 2,3-BD and acetoin were 13.9 and 13.3, respectively, compared to 13.4 and 12.7 obtained with the system consisting of 17% K2HPO4/21%ethanol (mass fraction). Little increase in multiple for 2,3-BD (NBD) occurred when the system was scaled up, indicating the concentration of product was stable during SOE process.
The partition behavior of residual reducing sugars was also studied at a 400-g scale. Most of the residual sugars were distributed to the bottom phase, as seen by the partition coefficients of residual sugars(which is the ratio of concentration of residual sugars in top phase to that in bottom phase) being less than 1[0.55±0.05 and 0.38±0.02 for 20% K2HPO4/19%ethanol (mass fraction) and 17% K2HPO4/21% ethanol (mass fraction), respectively]. The selective partition coefficient (KS) of 2,3-BD to reducing sugars were 23.7±0.3 and 34.0±0.1, respectively, indicating that 2,3-BD is more readily partitioned to the top phase compared to sugars. Therefore, SOE is an effective way to separate 2,3-BD from the residual sugars.
3.2 Removal of solid impurities and proteins
In this study the hydrolysate of Jerusalem artichoke stalk was directly subjected to fermentation without filtration, and milled powder of Jerusalem artichoke tuber was directly fed to the system during the fermentation process. Thus, a large amount of solid residues was present in the fermentation broth,as shown by the very high viscosity (Table 1). The viscosity of the fermentation broth was about 20-fold higher than that of the fermentation broth prepared from glucose (3.1 mPa?s) or filtered corn hydrolysate(3.3 mPa?s), and was contributed mainly by lignin,which was not removed from the hydrolysate of Jerusalem artichoke stalk.
The fermentation broth is usually filtered by membrane or centrifuged at high speed to remove cells and other solid residues before the isolation of target compounds. For Jerusalem artichoke-based fermentation broth, the traditional high-speed centrifugation (e.g. 6800×gfor 30 min) could not remove all the solid matters and the OD650of the broth was only decreased to 4.42. If the fermentation broth was directly filtered through a cellulose triacetate hollow fiber dialyzer, the membrane was quickly blocked.With SOE, these solid matters and part of the proteins were precipitated to the bottom phase or sequestered at the interphase. As a result, the values of OD650and viscosity were greatly lowered to 1.08 and 4.4 mPa?s,respectively, in the top phase (Table 1), meaning that 98% of the solid matters were removed from the top phase.
In the presence of high concentrations of solvent and salt, the soluble proteins were also precipitated at the interphase [9], decreasing from 7.75 g·L-1in thebroth to 1.86 g·L-1and 1.48 g·L-1, accounting for 87% and 89% soluble proteins removed from the top phases formed by 20% K2HPO4/19% Ethanol and 17%K2HPO4/21% Ethanol (mass fraction), respectively.Both were better than those achieved by a combination of centrifugation and membrane filtration (79.5%proteins removed), but close to the amount of protein(85.9%) removed from a glucose-based fermentation broth [9], and much better than that achieved with chitosan and polyacrylamide as flocculating agents(60% proteins removed) [17].
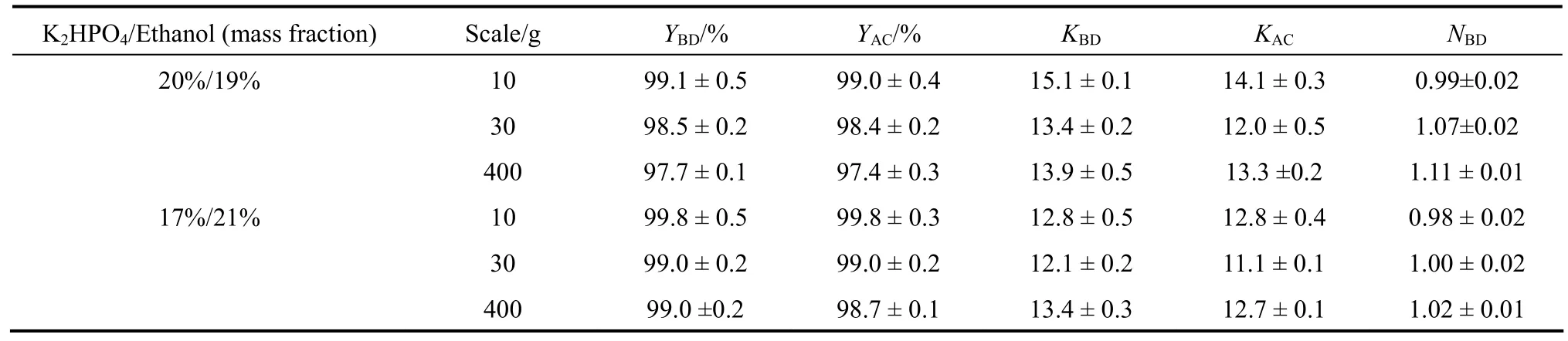
Table 2 Salting-out extraction under different scales using Jerusalem artichoke-based fermentation broth without pretreatment
SOE system requires high concentrations of organic solvent and inorganic salt, but they are much less than that used in solvent extraction or salting-out alone. Regardless of pretreated or untreated fermentation broth or high and low viscosity, high recovery of 2,3-BD was obtained, solid impurities and proteins were removed, and residual sugars were separated from 2,3-BD. The yield of 2,3-BD was much higher than that achieved by solvent extraction (75% by diethyl ether) [7] and slightly higher than that obtained with salting-out by K2CO3(96%-98%) [8]. In addition,SOE is also much simpler to operate. For fermentation broths having low viscosity, such as glucose-based broth, phases can be easily formed by allowing the mixture to stand for about 8-10 hours, while fermentation broth with a high viscosity may require a step of low-speed centrifugation to facilitate phase separation.SOE has clearly demonstrated its advantage over high-speed centrifugation or membrane filtration with gas sparging [5], and the incorporation of a low-speed centrifugation step in SOE has provided a much more practical and simpler way to separate 2,3-BD from viscous and solid-rich fermentation broths.
ACKNOWLEDGEMENTS
We gratefully thank Dr. Alan K. Chang for his help in revising the language of this paper.
1 Cheng, K.K., Liu, Q., Zhang, J.A., Li, J.P., Xu, J.M., Wang, G.H.,“Improved 2,3-butanediol production from corncob acid hydrolysate by fed-batch fermentation using Klebsiella oxytoca”,Process Biochem., 45 (4), 613-616 (2010).
2 Li, D., Dai, J.Y., Xiu, Z.L., “A novel strategy for integrated utilization of Jerusalem artichoke stalk and tuber for production of 2,3-butanediol byKlebsiella pneumoniae”,Bioresour.Technol., 101(21), 8342-8347 (2010).
3 Sun, L.H., Wang, X.D., Dai, J.Y., Xiu, Z.L., “Microbial production of 2,3-butanediol from Jerusalem artichoke tubers byKlebsiella pneumoniae”,Appl.Microbiol.Biotechnol., 82 (5), 847-852 (2009).
4 Anvari, M., Khayati, G., “In situ recovery of 2,3-butanediol from fermentation by liquid-liquid extraction”,J.Ind.Microbiol.Biotechnol., 36 (2), 313-317 (2009).
5 Gupta, B.S., Hashim, M.A., Ramachandran, K.B., Gupta, I.S., Cui,Z.F., “The effect of gas sparging in cross-flow microfiltration of 2,3-butanediol fermentation broth”,Eng.Life Sci., 5 (1), 54-57 (2005).
6 Shao, P., Kumar, A., “Recovery of 2,3-butanediol from water by a solvent extraction and pervaporation separation scheme”,J.Membr.Sci., 329 (1-2), 160-168 (2009).
7 Xiu, Z.L., Zeng, A.P., “Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol”,Appl.Microbiol.Biotechnol., 78 (6), 917-926 (2008).
8 Afschar, A.S., Vaz Rossell, C.E., Jonas, R., Quesada Chanto, A.,Schaller, K., “Microbial production and downstream processing of 2,3-butanediol”,J.Biotechnol., 27 (3), 317-329 (1993).
9 Jiang, B., Li, Z.G., Dai, J.Y., Zhang, D.J., Xiu, Z.L., “Aqueous two-phase extraction of 2,3-butanediol from fermentation broths using an ethanol/phosphate system”,Process Biochem., 44 (1), 112-117(2009).
10 Sun, L.H., Jiang, B., Xiu, Z.L., “Aqueous two-phase extraction of 2,3-butanediol from fermentation broths by isopropanol/ammonium sulfate system”,Biotechnol.Lett., 31 (3), 371-376 (2009).
11 van Soest, P.J., “Use of detergents in the analysis of fibrous feeds. II.A rapid method for the determination of fiber and lignin”,J.Ass.Offic.Agr.Chem., 46, 829-835 (1963).
12 van Soest, P.J., Robertson, J.B., Lewis, B.A., “Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition”,J.Dairy Sci., 74 (10), 3583-3597 (1991).
13 Li, Z., Teng, H., Xiu, Z., “Aqueous two-phase extraction of 2,3-butanediol from fermentation broths using an ammonium sulfate system”,Process Biochem., 45 (5), 731-737 (2010).
14 Li, Z., Jiang, B., Xiu, Z., “Aqueous two-phase extraction of 1,3-propanediol from glycerol-based fermentation broths”,Sep.Purif.Technol., 66 (3), 472-478 (2009).
15 Xu, X., “A Process of salt extraction with azeotropic distillation for extract lower water ethanol from fusel Oil”,Journal of Qinghai University(Natural Science Edition), 19 (4), 26-28 (2001).
16 Li, Z., Teng, H., Xiu, Z., “Extraction of 1,3-propanediol from glycerol-based fermentation broths with methanol/phosphate aqueous two phase system”,Process Biochem., 46, 586-591 (2011).
17 Hao, J., Xu, F., Liu, H.J., Liu, D.H., “Downstream processing of 1,3-propanediol fermentation broth”,J.Chem.Technol.Biotechnol.,81, 102-108 (2006).
 Chinese Journal of Chemical Engineering2011年4期
Chinese Journal of Chemical Engineering2011年4期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorptive Recovery of Uranium from Nuclear Fuel Industrial Wastewater by Titanium Loaded Collagen Fiber*
- Investigation of Mg2+/Li+ Separation by Nanofiltration*
- Vapor-Liquid Equilibrium of Ethylene + Mesitylene System and Process Simulation for Ethylene Recovery*
- Purification and Characterization of a Nonylphenol (NP)-degrading Enzyme from Bacillus cereus. Frankland*
- Sponge Effect on Coal Mine Methane Separation Based on Clathrate Hydrate Method*
- Modeling of Surface Tension and Viscosity for Non-electrolyte Systems by Means of the Equation of State for Square-wellChain Fluids with Variable Interaction Range*
