Different Outcome of Myeloid Sarcoma with Spinal Cord Compression Preceding Acute Myeloid Leukemia: Report of Two Cases and Review of Literatures
Qiang Yin, Yang-yang Zhou, Duo Chen, Wen-liang Li**
1Key Laboratory of Cancer Prevention and Therapy, Department of Neurosurgery and Neuro-oncology, Tianjin Medical University Cancer Hospital, Tianjin 300060, China;2Department of Neurosurgery, Shengjing Hospital Affiliated to China Medical University, Shenyang, China
Different Outcome of Myeloid Sarcoma with Spinal Cord Compression Preceding Acute Myeloid Leukemia: Report of Two Cases and Review of Literatures
Qiang Yin1, Yang-yang Zhou2*, Duo Chen2, Wen-liang Li1**
1Key Laboratory of Cancer Prevention and Therapy, Department of Neurosurgery and Neuro-oncology, Tianjin Medical University Cancer Hospital, Tianjin 300060, China;2Department of Neurosurgery, Shengjing Hospital Affiliated to China Medical University, Shenyang, China
Myeloid sarcomas (MS) preceding acute myeloid leukemia (AML) are rare, which presenting as acute spinal cord compression is even rare. Here we report two new cases of myeloid sarcoma patients, whose outcomes were different. Twenty-seven patients with spinal MS preceding AML have been reported to date, including the two cases presented in this article. Surgical decompression was performed in 25 of the 27 patients, and 23 of these
Spine; Myeloid sarcoma; Immunhistochemistry; Acute myeloid leukemia;
INTRODUCTION
MS can arise anywhere, but usually occur in the bone, skin, and lymph nodes[1]. Occurrence in the central nervous system and spinal cord is uncommon[2]. Once the incident occurs, the neurological dysfunction becomes worse and worse. So, emergency surgical decompression is an available treatment to make neural function recovery. To reduce the risk of subsequent AML in patients with nonleukemic MS, it is important to emphasize the need to treat patients who had nonleukemic MS with AML-type therapy, except of resection or irradiation[3,4]. Herein, we report two cases of patients with MS with spinal cord compression preceding AML. They accepted different therapies and their outcomes were different.
CASE REPORT
Case One
A 28-year-old previously healthy man presented with progressive low back pain and numbness of his legs for 10 days and bladder incontinence for 1 day. At physical examination, he was awake, alert, and fully oriented. His cranial nerves were intact. Sensory assessment revealed hypoesthesia below L12 and motor examination revealed 2/5 paraparesis. Deep tendon reflexes were hyperactive and Babinski signs were bilaterally positive. Laboratory evaluation revealed a white blood cell count of 3,900/mm3, a hemoglobin level of 11.0 g/dl and a hematocrit of 38.2%. Magnetic resonance imaging of the spine revealed a posterior epidural mass between T12 and L1 (Figure 1). High-dose methylprednisolone was given initially, and he underwent emergent spinal cord decompression with T12-L1 laminectomy and tumor resection. A soft and grayish tumor compressing thespinal cord was identified. Histologically the tumor was composed of a relatively uniform population of immature cells (Figure 2A). Immunohistochemical staining revealed the expressions of CD68, CD45, CD43, CD117 and lysozyme but not of MPO, CD20(Figure 2 B-H). The result suggested a diagnosis of MS in vertebral canal. There was nothing wrong with both bone marrow biopsy and his blood. After 10 days he gradually gained strength in his lower extremities and his bladder function was recovered. But the patient refused standard chemotherapy and radiation therapy to the spinal axis and tumor bed. After 4 months he presented with progressive low back pain and a high fever. At that time we found immature monoblasts from his blood (Figure 3A). And bone marrow biopsy results were consistent with acute myelocytic leukemia. The results of bone marrow slides showed that bone marrow hyperplasia was supreme, and most of the myeloid cells were immature monoblasts (Figure 3B). The patient was treated with anti-AML therapy, but died of sepsis finally.
Case Two
A 20-year-old girl presented with progressive back pain and numbness of her legs for 3 days. Sensory assessment revealed hypoesthesia below T7 and motor examination revealed 3/5 paraparesis. She was unable to stand or walk independently. Deep tendon reflexes were hyperactive and Babinski signs were bilaterally positive. Laboratory evaluation revealed a white blood cell count of 4,900/mm3, a hemogloin level of 12.2 g/dl and a hematocrit of 37.2%. Magnetic resonance imaging of the thoracic spine revealed a posterior epidural mass between T7 and T9 (Figure 4). She underwent emergent spinal cord decompression with T7-T9 and tumor resection. A soft tumor compressing the spinal cord was identified. Histologically the tumor was composed of a relatively uniform population of immature cells, most of them were monocaryon (Figure 5A). Immunohistochemical staining revealed the expressions of CD68, CD45, and lysozyme, but not of MPO, CD20 (Figure 5B-F). These findings were consistent with a diagnosis of MS. There was nothing wrong with both bone marrow biopsy and her blood. After 9 days she gradually gained strength in his lower extremities. The patient accepted standard chemotherapy and radiation therapy to the spinal axis and tumor bed. During the 20 months follow-up she was with no evidence of leukemia disease, and there was nothing wrong with results of her three bone marrow biopsy.
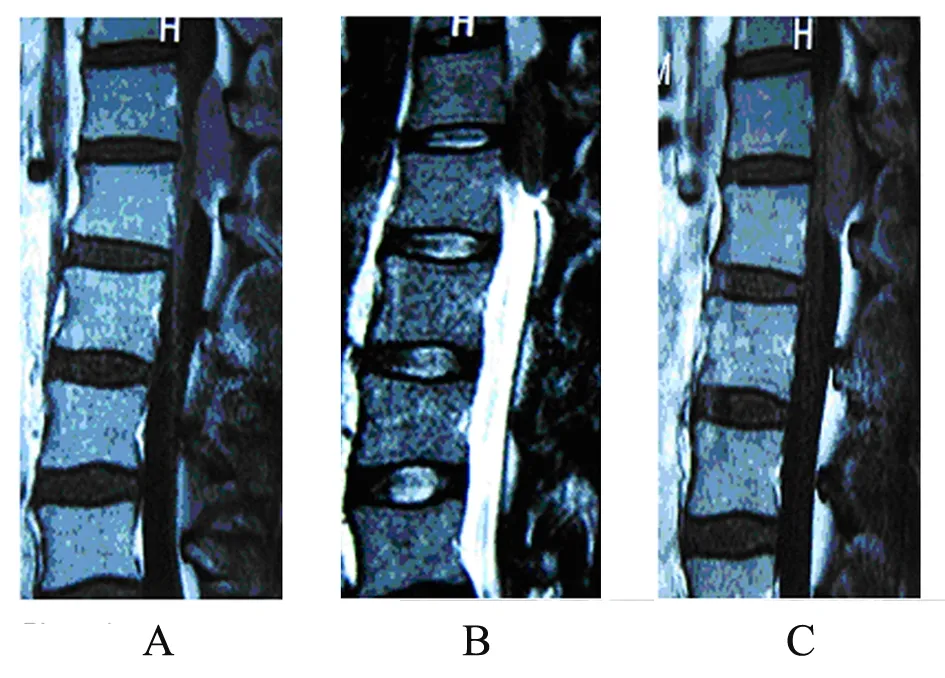
Figure 1. MRI studies at the level of the T12-L1 disc space demonstrating the posterior epidural mass with near total obliteration of the spinal canal. (A)∶ T1-weighted MRI scan; (B)∶ T2-weighted MRI scan; (C)∶ T1-weightd gadoliniumenhanced MRI scan.
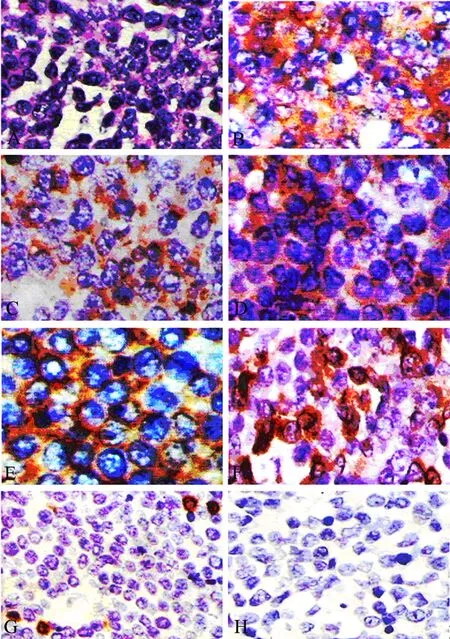
Figure 2. The histological and immunohistochemical study (case one). (A)∶ Micrograph showing a meyloid sarcoma composed of atypical cells (Hematoxylin and eosin stain ×400); (B-H)∶ Photomicrographs demonstrating positive immunohistochemical staining for CD68(B), CD45(C), CD43(D), CD117(E), Lysozyme(F) and negative for MPO(G), CD20(H).
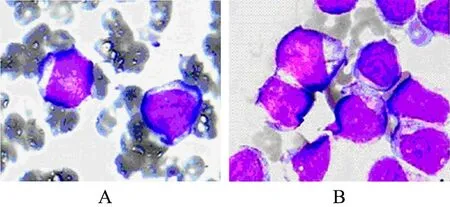
Figure 3. photomicrograph showing immature mono- blasts in peripheral blood. (A) and in bone marrow (B).
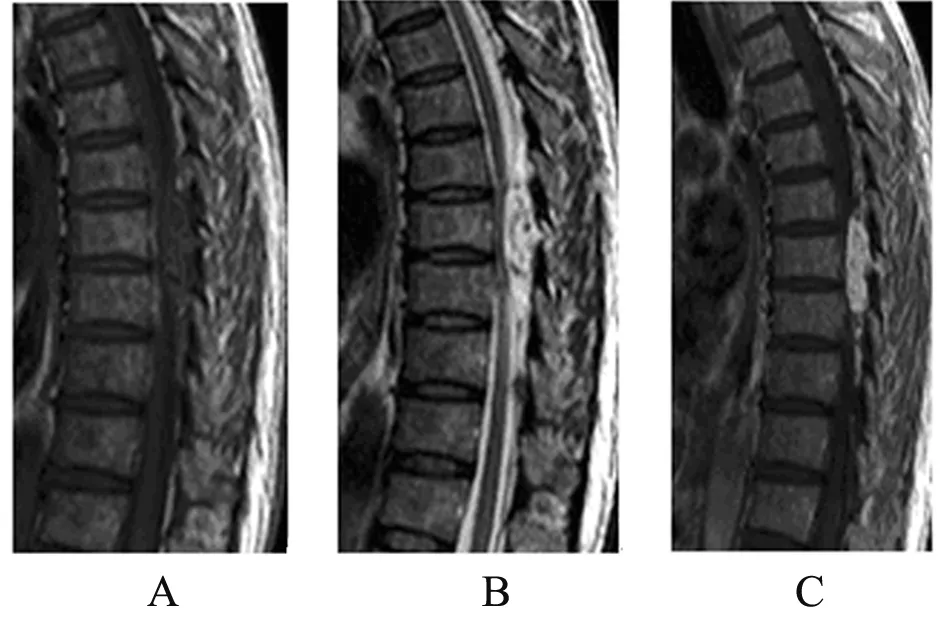
Figure 4. MRI studies at the level of the T7-T9 disc space demonstrating the posterior epidural mass with near total obliteration of the spinal canal. (A)∶ T1-weighted MRI scan; (B)∶ T2-weighted MRI scan; (C) T1-weighted gadoliniumenhanced MRI scan.
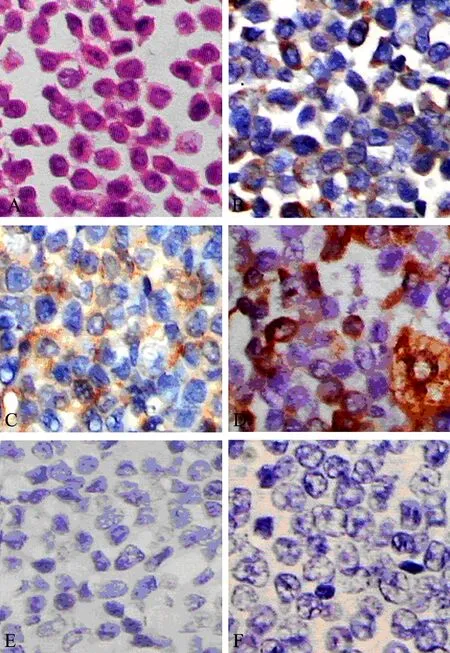
Figure 5. The histological and immunohistochemical study (case two). (A)∶ Micrograph showing a myeloid sarcoma composed of atypical cells. (Hematoxylin and eosin stain ×400) (B-I)∶ Photomicrographs demons- trating positive immunohistochemical staining for CD68(B), CD45(C), Lysozyme(D) and negatve for MPO(E), CD20(F).
DISCUSSION
Twenty-seven patients with spinal myeloid sarcoma preceding AML, 23 men and 4 women aged 2 to 74 years ( median, 32 years), have been reported to date, including the two cases presented in this article[2,5-27](Table1). The affected sites included 2 at cervical, 19 at thoracic, 10 at lumbar, and 4 at sacral spinal levels. The chief clinical symptoms were related to the affected sites. MS is a rare, solid, malignant extramedullary tumor of myeloid cells. MS is seen more often in men than in women as is generally the case in leukemia[18]. The male predominance could be associated with the higher incidence of acute myeloid leukemia in men[28]. The tumor may appear at any age or localisation, and usually occurs in the subperiost of the vertebra, sternum, orbits and skull. The involvement of the central nervous system and spinal cord is rare, myelopathy due to tumor compression is even rarer[29]. Most spinal myeloid sarcomas developed at the thoracic level, followed respectively by the lumbar, sacral and cervical level[30].
Immunohistochemical study played an essential role in arriving at a correct diagnosis of myeloid sarcoma. Nineteen of the spinal myeloid sarcomas were correctly diagnosed by immunohistochemical staining, including the two cases presented in this article[5,7-10,12-14,17-20,25,26]. The correct and prompt diagnosis of MS was important for adequate therapy, which was often delayed because of a high misdiagnosis rate[31]. The histological diagnosis of MS is difficult in the absence of evidence of AML. The differential diagnosis includes Ewing sarcoma, Langerhans histiocytosis, B-cell or T-cell lymphoma and metastatic tumors[1,32]. With extensive morphological and immunohistochemical analyses, the myeloid sarcoma were classified into five types∶a) immature granulocytic sarcoma; b) differentiated granulocytic sarcoma; c) monoblastic sarcoma; d) monocytic myeloid sarcoma, and e) myelomonocytic sarcoma[15]. An immunohistochemical staining including lysozyme, CD43, CD45, MPO, CD117, CD68, CD3 and CD20 can successfully identify the majority of MS in formalin-fixed, paraffin-embedded tissue specimens[33-37]. The most common type of MS is the granulocytic sarcoma composed of myeloblasts and neutrophils. The less common type, monoblastic sarcomas have similar surface antigens to that of monoblasts in acute monoblastic leukemias. CD43 and lysozyme are the most sensitive markers staining a large proportion of neoplastic cells in all tumors examined. MPO and CD117 are the most sensitive of the markers for myeloid differentiation while monocytic precursors consistently strongly expressed CD68 and CD163[38].
Surgery is generally preferred for cases of acute spinal cord compression. Surgical decompression was performed in 25 of the 27 patients, including the two cases presented[5-21,24-27]. MS is a malignant tumor, and its growth speed is fast. When the tumor occurred in the small intraspinal space, it could result in spinal cord compression, and spinal cord function was damaged in a very short time. So emergency decompression is an available treatment to make neural function recovery, which is associated with high survival quality.
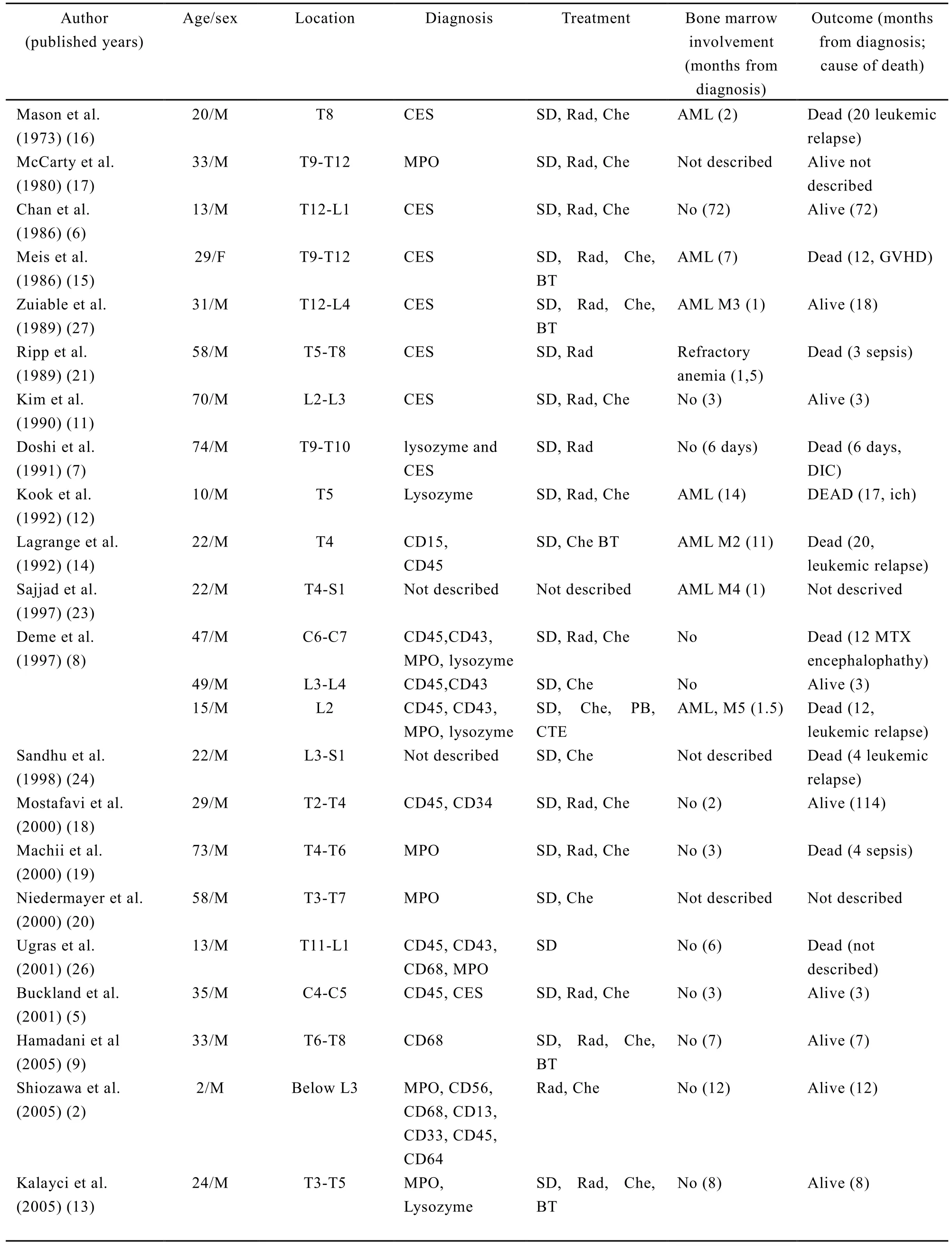
Table 1. Summary of clinical findings in patients with non-leukemic spinal myeloid sarcoma
CES∶ chloroacetate esterase staining; AML∶ acute myeloid leukemia; BT∶ bonemarrow transplantation; Che∶chemotherapy; DIC∶ disseminated intravascular coagulation disorder; GVHD∶ graft versus host disease; ICH∶intracranial hemorrhage; MTX∶ methotrexate; PBSCT∶ peripheral blood stem cell transplantation; Rad∶radiotherapy, SD∶ surgical decompressing; MPO∶ (myeloperoxidese) is a poroxidase enzyme most abundantly present in neutrophil granulocytes.
However, local treatments for MS, such as radiation therapy or surgical resection, are less effective than chemotherapy at improving the disease-free interval or disease-free survival. MS occurs most commonly in patients with previous or current AML or in patients with chronic myeloproliferative disorders as a sign of blast transformation[39,40]. MS may be also encountered in patients with no history of hematologic disorder[15]. For patients without leukemia, MS is frequently a herald for AML. In one study, 71% of the patients diagnosed with MS who did not receive antileukemic chemotherapy subsequently developed AML within a median time of 7 months[41]. A review of nonleukaemic MS concluded that chemotherapy used for acute non-lymphoblastic leukaemia was more effective than local treatment modalities specifically directed at myeloid sarcoma[3]. A current study demonstrated that anti-AML therapy is highly effective in patients with nonleukemic MS and is associated with higher rates of event-free survival and overall survival in MS than in AML after matching MS patients with comparable, typical AML patients[4]. Bonemarrow transplantation has also shown promise. Some cases of primary myeloid sarcoma treated with autologous bonemarrow transplantation secondary to radical surgery, chemotherapy, and local prophylactic irradiation, has also been reported to have a long period of disease-free survival[25,42].
Considering our patients and the published cases in the literature we suggest that immunohistochemical study plays an essential role in arriving at a correct diagnosis of myeloid sarcoma, and the combination therapy of emergency surgery and anti-AML therapy is an available treatment to spinal MS preceding AML.
REFERENCES
[1] Brunning RD, Matutes E, Fladrin G, et al. Acutemyeloid leukaemia not otherwise categorised. In∶ Jaffe ESHN, SteinH, Vardiman JW, editors. World Health Organization classification of tumors. Pathology and genetics of tumors of haematopoieticand lymphoid tissues[D]. Lyon∶ IARC Press, 2001; 104-5.
[2] Shiozawa Y, Kiyokawa N, saito M, et al. Granuocytic sarcoma of the spine in a child without bone marrow involvement∶ a case report and literature review[J]. Eur J Pediatr 2005; 164∶ 616-20.
[3] Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma∶ report of two cases and a review of 72 cases in the literature[J]. Cancer 2002; 94∶1739-46.
[4] Tsimberidou AM, Kantarjian HM, Wen S, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia[J]. Cancer 2008; 113∶1370-8.
[5] Buckland ME, Scolyer RA, Donellan MB, et al. Spinal chloroma presenting with triplegia in an aleukaemic patient[J]. Pathology 2001; 33∶ 386-9.
[6] Chan JK, Lau WH, Saw D. Extradural granulocytic sarcoma of the spine∶ a unique case of long survival after local therapy[J]. Am J Hematol 1986; 22∶439-41.
[7] Doshi HM, Schochet SS Jr, Gold M, et al. Granulocytic sarcoma presenting as an epidural mass with acute paraparesis in an aleukemic patient[J]. Am J Clin Pathol 1991; 95∶ 228-32.
[8] Deme S, Deodhare SS, Tucker WS, et al. Granulocytic sarcoma of the spine in nonleukemic patients∶ report of three cases[J]. Neurosurgery 1997; 40∶ 1283-7.
[9] Hamadani M, Tfayli A, Sethi S, et al. Granulocytic sarcoma manifesting as multiple skeletal lesions[J]. Am J Med Sci 2005; 330∶ 139-43.
[10] Howell DL, Abramowsky CR. Granulocytic sarcoma presenting as a large mediastinal mass with acute spinal cord compression in a non-leukemic child[J]. Pediatr Blood Cancer 2005; 44∶ 527.
[11] Kim FS, Rutka JT, Bernstein M, et al. Intradural granulocytic sarcoma presenting as a lumbar radiculopathy. Case report[J]. J Neurosurg 1990; 72∶663-7.
[12] Kook H, Hwang TJ, Choe K, et al. Spinal epidural granulocytic sarcoma preceding acute myelogenous leukemia[J]. J Korean Med Sci 1992; 7∶ 291-6.
[13] Kalayci M, Sumer M, Yenidunya S, et al. Spinal granulocytic sarcoma (chloroma) presenting as acute cord compression in a nonleukemic patient[J]. Neurol India 2005; 53∶ 221-3.
[14] Lagrange M, Gaspard MH, Lagrange JL, et al. Granulocytic sarcoma with meningeal leukemia but no bone marrow involvement at presentation. A report of two cases with characteristic cerebrospinal fluid cytology[J]. Acta Cytol 1992; 36∶ 319-24.
[15] Meis JM, Butler JJ, Osborne BM, et al. Granulocytic sarcoma in nonleukemic patients[J]. Cancer 1986; 58∶2697-709.
[16] Mason TE, Demaree RS Jr, Margolis CI. Granulocytic sarcoma (chloroma), two years preceding myelogenous leukemia[J]. Cancer 1973; 31∶ 423-32.
[17] McCarty KS Jr, Wortman J, Daly J, et al. Chloroma (granulocytic sarcoma) without evidence of leukemia∶facilitated light microscopic diagnosis[J]. Blood 1980; 56∶ 104-8.
[18] Mostafavi H, Lennarson PJ, Traynelis VC. Granulocytic sarcoma of the spine[J]. Neurosurgery 2000; 46∶ 78-84.
[19] Machii R, Muto A, Okano Y, et al. Granulocytic sarcoma presenting as an epidural mass with spinal cord compression[J]. Rinsho Ketsueki 2000; 41∶653-7.
[20] Niedermayer I, Schmitt-Graeff A, Kolbel CB, et al. Granulocytic sarcoma (so-called chloroma) as a possible cause of spinal cord compression. Case report and differential diagnosis[J]. Pathologe 2000; 21∶ 82-5.
[21] Ripp DJ, Davis JW, Rengachary SS, et al. Granulocytic sarcoma presenting as an epidural mass with cord compression[J]. Neurosurgery 1989; 24∶125-8.
[22] Alexiev BA, Wang W, Ning Y. Myeloid sarcomas∶ a histologic, immunohistochemical, and cytogenetic study[J]. Diagn Pathol 2007; 2∶ 42.
[23] Sajjad Z, Haq N, Kandula V. Case report∶granulocytic sarcoma (GS) presenting as acute cord compression in a previously undiagnosed patient[J]. Clin Radiol 1997; 52∶ 69-71.
[24] Sandhu GS, Ghufoor K, Gonzalez-Garcia J, et al. Granulocytic sarcoma presenting as cauda equina syndrome[J]. Clin Neurol Neurosurg 1998; 100∶205-8.
[25] Inoue T, Takahashi T. Spinal granulocytic sarcoma manifesting as radiculopathy in a nonleukemic patient[J]. Neurol Med Chir 2008; 48∶131-6.
[26] Ugras S, Cirak B, Karakok M, et al. Spinal epidural granulocytic sarcoma (chloroma) in a non-leukemic child[J]. Pediatr Int 2001; 43∶ 505-7.
[27] Zuiable A, Aboud H, Nandi A, et al. Extramedullary disease initially without bone marrow involvement in acute promyelocytic leukaemia[J]. Clin Lab Haematol 1989; 11∶ 288-9.
[28] Cartwright RA, Gurney KA, Moorman AV. Sex ratios and the risks of haematological malignancies[J]. Br J Haematal 2002; 118∶1071-7.
[29] Widhalm G, Dietrich W, Mullauer L, et al. Myeloid sarcoma with multiple lesions of the central nervous system in a patient without leukemia. Case report[J]. J Neurosurg 2006; 105∶ 916-9.
[30] Landis DM, Aboul fia a DM. Granulocytic sarcoma∶ an unusual complication of aleukemic myeloid leukemia causing spinal cord compression. A case report and literature review[J]. Leuk Lymphoma 2003; 44∶1753-60.
[31] Colella G, Tirelli A, Capone R, et al. Myeloid sarcoma occuring in the maxillary gingiva∶ a case without leukemic manifestations[J]. Int J Hematol 2005; 81∶ 138-41.
[32] Zekry N, Klooster MJ, Raghavan R, et al. A 7-year-old child with a history of acute myeloidleukemia presenting with multiple gastrointestinal polyps[J]. Arch Pathol Lab Med 2006; 130∶ 3-4.
[33] Li JM, Liu WP, Zhang MH, et al. Clinicopathologic and immunophenotypic analysis of myeloid sarcoma[J]. Zhonghua Bing Li Xue Za Zhi(in Chinese) 2006; 35∶ 606-11.
[34] Liu YH, Zhuang HG, Liao XB, et al. Diagnosis and differential diagnosis of granulocytic sarcomas[J]. Zhonghua Xue Ye Xue Za Zhi (in Chinese) 2003; 24∶568-71.
[35] Chang CC, Eshoa C, Kampalath B, et al. Immunophenotypic profile of myeloid cells in granulocytic sarcoma by immunohistochemistry. Correlation with blast differentiation in bone marrow[J]. Am J Clin Pathol 2000; 114∶ 807-11.
[36] Hudock J, Chatten J, Miettinen M∶ Immunohistochemical evaluation of myeloid leukemia infiltrates (granulocytic sarcomas) in formaldehydefixed, paraffin-embedded tissue[J]. Am J Clin Pathol 1994; 102∶ 55-60.
[37] Traweek ST, Arber DA, Rappaport H, et al. Extramedullary myeloid cell tumors. An immunohistochemical and morphological study of 28 cases[J]. Am J Surg Pathol 1993; 17∶ 1011-9.
[38] Menasce LP, Banerjee SS, Beckett E, et al. Extramedullary myeloid tumor (granulocytic sarcoma) is often misdiagnosed∶ a study of 26 cases[J]. Histopathol 1999; 34∶ 391-8.
[39] Garcia MG, Deavers MT, Knoblock RJ, et al. Myeloid sarcoma involving the gynecologic tract∶ a report of 11 cases and review of the literature[J]. Am J Clin Pathol 2006; 125∶ 783-90.
[40] Muss HB, Moloney WC. Chloroma and other myeloblastic tumors[J]. Blood 1973; 42∶ 721-8.
[41] Imrie KR, Kovacs MJ, Selby D, et al. Isolated chloroma∶ the effect of early antileukemic therapy[J]. Ann Intern Med 1995; 123∶ 351-3.
[42] Stevenson WS, Vincent PC, Iland HJ. Disseminated granulocytic sarcoma treated with allogeneic peripheral blood stem-cell transplantation[J]. Leuk Lymphoma 2002; 43∶ 2221-4.
R733.7 Document code: A Article ID: 1000-9604(2010)02-0156-07
10.1007/s11670-010-0156-y
rticle, 21 of the 27 patients received anti-AML therapy, consisting of chemotherapy, or bone marrow transplantation, including the one case presented , and the survival time was 3 to72 months (median, 14 months)[5,6,8-20,22,24,25,27]. Between the two new presented cases there were some similar pionts, but their outcomes were very different. They presented with back pain as the first symptom, and developed into spinal cord dysfunction at a short period of time. Their same immunohistochemical staining revealed the expressions of CD68, CD45, and lysozyme. We did not find any leukemia cells in their bone marrow at first. But one refused chemotherapy, and developed into AML after 4 months, and died of sepsis finally. The other case accepted the standard anti-AML therapy, and during 20 months follow-up she was still alive. The totally different outcome may be associated with whether or not accepting the anti-AML therapy.
Received 2009?08?21; Accepted 2010?01?12*Contributed equally to this study.
**Corresponding author.
E-mail∶ lwlyq1982@126.com
additional anti-AML therapy. Considering our patients and the published cases in the literature we suggest that immunohistochemical study plays an essential role in arriving at a correct diagnosis of MS, and that emergency surgery to resect spinal MS is an available treatment to make neural function recovery, and that the disease must be treated with intensive chemotherapy similar to that used to treat AML as soon as possible after resection or irradiation of the tumor.
? Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2010
 Chinese Journal of Cancer Research2010年2期
Chinese Journal of Cancer Research2010年2期
- Chinese Journal of Cancer Research的其它文章
- Triptolide Inhibits Cell Growth and Induces G0- G1 Arrest by Regulating P21wap1/cip1 and P27 kip1 in Human Multiple Myeloma RPMI-8226 Cells
- Effects of Triptolide on Histone Acetylation and HDAC8 Expression in Multiple Myeloma in vitro
- CGI-100 Specific shRNA Inhibits Proliferation and Induces Differentiation in Leukemia K562 Cells
- Expression of Embryonic Stem Cell Marker Oct-4 and Its Prognostic Significance in Rectal Adenocarcinoma
- Differential Diagnosis of Warthin's Tumor Complicated with Lung Adenocarcinoma by 18F- FDG PET/CT Imaging and Radioisotope Scanning with Tc-99m Pertechnetate: A Case Report and Literature Review
- Synergistic Action of fMLP-boanmycin Combination on the Growth of Mouse Colon Carcinoma and Its Action Mechanisms
